Breast Cancer Screening in 2021
Dr Pierre Vassallo
October is Breast Cancer Awareness month, which is an annual worldwide campaign aimed to increase awareness of the disease and to stress the importance of screening, early diagnosis, and prompt treatment.
Why is screening for breast cancer so important? Primarily because after skin cancer, breast cancer is the most common cancer in women affecting 1 in 8 individuals. It is therefore the cause of much suffering particularly amongst women and may cause early death. Breast cancer screening detects cancer early allowing more effective management with less aggressive therapy. Early and effective treatment results in a better quality of life and less stress for family members and loved ones. Screening of family members of women suffering from breast cancer particularly siblings and twins, is recommended to detect early disease in these individuals and to prevent complications.
The currently available breast cancer screening tests are Ultrasound, Mammography and MRI. However, each of these modalities consists of a number of different technologies. In the following paragraphs, we will describe each technology and list its advantages and limitations.
- Ultrasound
There are two types of breast ultrasound, Handheld Breast Ultrasound (HHBUS) and Automated Breast Ultrasound (ABUS).
HHBUS
HHBUS is the standard screening test for women less than 40 years of age. It is also used as a supplemental exam with mammography for those who are 40 years and older, particularly for those women who have dense breasts.1 Combining HHBUS with mammography results has significant advantages for breast cancer detection, which will be discussed in more detail below.
If a lesion is found with HHBUS, it can be further evaluated with Ultrasound Elastography. Shear Wave Elastography has been shown to be valuable in distinguishing benign from cancerous nodules.2 However, irrespective of findings on Elastography, one must proceed to ultrasound-guided biopsy for more reliable confirmation.
HHBUS is operator-dependent and achieves greater accuracy in the hands of an experienced breast radiologist. For patients presenting with a palpable nodule, HHBUS is the best evaluation tool, allowing for rapid treatment decisions on whether biopsy is required (Fig 1).
ABUS
ABUS was conceived specifically to address the operator dependence of HHBUS. As its name states, it is an ultrasound machine that obtains a 3D volumetric image of the breast, which can later be reviewed by the radiologist. ABUS lacks the flexibility of targeted analysis with free selection of scan plane that is possible with HHBUS in the hands of an experienced radiologist. ABUS has other limitations particularly for covering large breasts, where supplemental HHBUS may be required. It is associated with long interpretation times and results in an increased number of recalls for reassessment with HHBUS. ABUS has lower sensitivity but higher specificity than HHBUS, but overall it has no demonstrable statistical advantage over HHBUS.1
- Mammography
There are different mammographic technologies available on the market, which differ in their diagnostic accuracy. We will discuss Computed Mammography (CM), Digital Mammography (DM), Digital Breast Tomosynthesis (DBT) and contrast-enhanced Mammography (CEM).
Computed Mammography
CM, also known as CR (computed radiographic) mammography, is old technology that is not truly digital. It uses a CR plate to take a breast image that is then read through a dedicated scanner. It is listed here because it is still widely available in breast cancer screening clinics. It is inferior to DM and DBT mammography for the detection of breast cancer. CM has been shown to be 21% less effective in detecting breast cancers than DM.3 In fact, some authors go as far as recommending that patients should be advised before performing CM that the test has a lower cancer detection rate.4
Digital Mammography
DM (sometimes called 2D mammography) is a well-establish accurate technology that is the mainstay for breast cancer screening programs. Images of the breast are obtained through a special solid-state detector and transferred to a diagnostic workstation, where they are reviewed by a radiologist (Fig 2). DM is frequently adequate alone as a screening tool for breast cancer. With equivocal findings or in case of dense breasts, supplemental exams such as HHBUS or DBT may be required.
Digital Breast Tomosynthesis
DBT (sometimes incorrectly referred to as 3D mammography) is a technological enhancement of DM. DBT delivers 1mm thick slices through the breast, the number of which varies depending on breast thickness. Thus, rather than having one image in each plane as with DM, as many as 85 images per plane may be delivered by DBT. Scrolling through such a stack of images is like flipping through the pages of a book; it allows detailed analysis at different depths of the breast. This is particularly useful with dense breasts, where overlying dense glands may hide a small breast cancer.
DBT has the added advantage of reducing the requirement for additional views such as magnified and spot compression views, thus reducing radiation exposure.
Synthesised Mammograms (SM) are single images of the breast generated from a DBT dataset. While they may appear similar to DM, they are not a replacement from an accuracy standpoint. SMs must be used as a rapid overview of the breast before proceeding with more detailed evaluation of the DBT images.
DBT has the advantage of detecting the architectural distortion, which is an indicator of invasive cancers. It therefore preferentially improves the detection rate of invasive ductal and lobular cancers without increasing that of in-situ cancers.5 DBT therefore maximises the detection of biologically significant disease and prevents over-diagnosis. DBT is particularly valuable for detecting invasive lobular cancers, which are normally known to be elusive or occult on DM. DBT improves conspicuity of lesion margins (Fig 3).
It is important to note, that not all architectural distortions are due to breast cancer. In fact, 33.2% of architectural distortions seen on DBT are radial scars.6 However, even biopsy-confirmed radial scars are best excised since analysis of the excised lesion may upgrade it to cancer.
The fact that DBT improves cancer detection rate over DM has been demonstrated in a number of large studies (Fig 4). In one large population-based study, cancer detection rates in women who underwent consecutive biennial screening with DM after DM, DBT after DM, and DBT after DBT were 4.6, 9.9, and 8.3 per 1000 examinations, respectively.7
Detection rate of breast cancer is highest on the first DBT; the statistical reason for this is that women coming for follow-up mammograms have already been previously screened for breast cancer.
Follow-up DBT detects interval cancers; these are cancers that develop in the interval between one mammogram and the next. These are aggressive cancers, because their rate of growth has outpaced the frequency of screening mammograms (recommended yearly). Their early detection prevents progression to advanced cancers; these are defined as those with metastases, positive lymph nodes, invasive tumours >2cm in diameter, and oestrogen/progesterone receptor negative or Herceptin receptor-positive invasive cancers >1cm in diameter.8
Contrast Enhanced Mammography
CEM is a new technology that is still under investigation. It uses images taken with two X-ray energies to obtain mammograms that are sensitive to injected contrast medium. In some respect, it is similar to contrast-enhanced MRI since cancer detection is based on uptake of intravenously injected contrast material. Early reports are suggesting that it has similar accuracy. CEM may be combined with DBT technology to improve lesion conspicuity.9
- MRI
Breast MRI is the most sensitive technology for the detection of breast cancer with a sensitivity (up to 96%) reported as being higher than that of mammography and ultrasound (Fig 5). It is specifically recommended for women with a high risk for breast cancer, such as those with inherited genetic mutation and those who have had mantle radiation to their breasts before 30 years of age.10
Breast MRI is based on functional imaging that detects breast cancer based on its increased vascularity and contrast enhancement. This modality detects invasive and high-grade cancers.11
Breast MRI using the standard protocol is not practical as a screening tool, because of the long exam times. It is best used as a supplemental exam for DBT or DM, when findings on these modalities are equivocal.
The development of an abbreviated MRI protocol has been discussed in recent years and appears to be gaining interest. However, the exam still takes longer to perform than DBT or DM. A recent comparison between Abbreviated Breast MRI and DBT has shown that MRI is more sensitive, but DBT is more specific for breast cancer detection.12
Multimodality Screening
When evaluating the accuracy of a screening service, it is important to appreciate that combining modalities increases the rate of cancer detection and has an impact on treatment outcome.
Supplemental HHBUS has been shown to improve the sensitivity for breast cancer detection in women with extremely dense breasts13 (Fig 6). This is particularly important since women with extremely dense breasts have a 4-6 times higher risk of developing breast cancer.14
Studies have shown that supplementing DM with HHBUS increases cancer detection rates. There is also an incremental gain in cancer detection rate with each year of screening.13 Cancers detected with DM with supplemental HHBUS tended to be small, invasive and node negative.13 Supplemental HHBUS has been shown to lower the interval cancer detection rate.15
HHBUS diminishes the false positive recall rate; this is the rate at which patients who are called back for further imaging turn out to be disease negative.
One question that remains to be answered is whether DBT can fully replace the combination of HHBUS and DM. In clinical practice, using DBT results in less need for supplemental HHBUS.
Supplemental breast MRI detected additional cancers when performed after DM and HHBUS.13 In extremely dense breasts, a number of studies are recommending proceeding directly to supplemental MRI rather than performing HHBUS.
In case of extremely dense breasts, even with DBT, supplemental evaluation with HHBUS and MRI may still be required. Combining these modalities has been shown to increase the detection rate of invasive cancer.
The complexity of current breast cancer screening options is daunting. Studies are constantly revealing new knowledge that helps us establish a standard protocol. However, it is becoming increasingly clear that this protocol will need to be tailored to patient needs based on cancer risk and to biological subtype.
Current Standard Protocol
- Women 40 years and over should have yearly mammograms (DBT preferred over DM) and supplemental imaging as deemed necessary by the breast radiologist.
- Between the ages of 30 and 40 years, women should have a yearly HHBUS if they have a strong family history of any cancer especially breast and ovarian cancers.
- Positive breast cancer gene (BRACA1/2) women should have yearly breast MRI scans especially when they have dense breasts.
Figure Legends
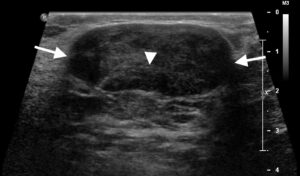
Figure 1. HHBUS showing a flat oval well-defined nodule (arrows) with a central echogenic line (arrowhead) in a 28 year old woman; all features are indicative of a fibroadenoma.
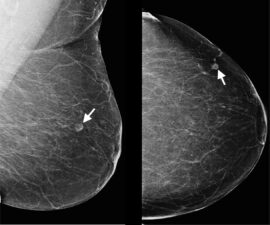
Figure 2. DM showing a small lesion in the lateral portion of the left breast that contains central hilar fat; the fatty hilum was also noted on HHBUS confirming the lesion to be an intramammary lymph node. Note the high quality of the images delivered by DM that cover the whole breast in each view.
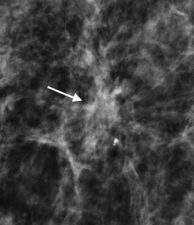
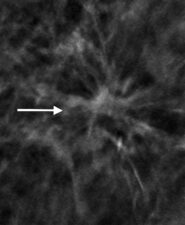
Figure 3. (a) Invasive ductal cancer detected on DM; however, margins of the lesion are better detected on DBT (b).


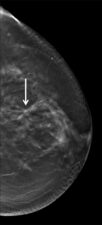
Figure 4. Occult invasive ductal cancer on mediolateral oblique (a) and bilateral craniocaudal (b) DMs, but visible on bilateral craniocaudal DBT (c).
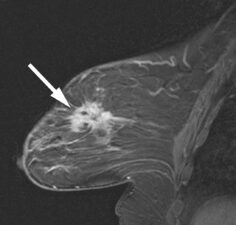
Figure 5. Sagittal contrast enhanced T1-w breast MRI scan showing an invasive ductal cancer (arrow).
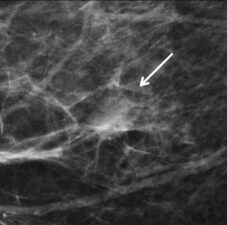
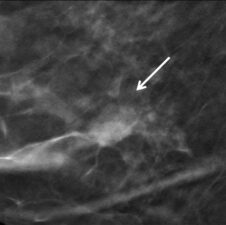
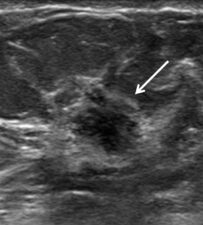
Figure 6. Invasive ductal cancer (arrow) seen on DM (a) and in-plane DBT (b). HHBUS confirmed a spiculated lesion (arrow) at the site and subsequently helped guide core biopsy.
References
- Lin X, Jia M, Zhou X, et al. The diagnostic performance of automated versus handheld breast ultrasound and mammography in symptomatic outpatient women: a multicenter, cross-sectional study in China. Eur Radiol 2021;31(2):947-957.
- Au FW, Ghai S, Moshonov H, et al. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. AJR 2014;203(3):W328-36.
- Yaffe MJ, Bloomquist AK, Hunter DM, et al. Comparative performance of modern digital mammography systems in a large breast screening program. Med Phys 2013;40(12):121915.
- Chiarelli AM, Edwards SA, Prummel MV, et al. Digital compared with screen-film mammography: performance measures in concurrent cohorts within an organized breast screening program. Radiology 2013;268(3):684-93.
- Gao Y, Moy L, Heller SL. Digital Breast Tomosynthesis: Update on Technology, Evidence, and Clinical Practice. Radiographics 2021;41(2):321-337.
- Vijayaraghavan GR, Newburg A, Vedantham S. Positive Predictive Value of Tomosynthesis-guided Biopsies of Architectural Distortions Seen on Digital Breast Tomosynthesis and without an Ultrasound Correlate. J Clin Imaging Sci 2019;9:53.
- Hovda T, Brandal SHB, Sebuødegård S, et al. Screening outcome for consecutive examinations with digital breast tomosynthesis versus standard digital mammography in a population-based screening program. Eur Radiol 2019;29(12):6991-6999.
- Lewin J. Comparison of Contrast-Enhanced Mammography and Contrast-Enhanced Breast MR Imaging. Magn Reson Imaging Clin N Am 2018;26(2):259-263.
- Pisano ED. Is Tomosynthesis the Future of Breast Cancer Screening? Radiology 2018;287(1):47-48.
- Maxwell AJ, Lim YY, Hurley E, et al. False-negative MRI breast screening in high-risk women. Clin Radiol 2017;72(3):207-216.
- Sung JS, Stamler S, Brooks J, et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016;280(3):716-22.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 2020;323(8):746-756. Erratum in: JAMA 2020;323(12):1194.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394-404.
- Rafferty EA, Durand MA, Conant EF, et al. Breast Cancer Screening Using Tomosynthesis and Digital Mammography in Dense and Nondense Breasts. JAMA 2016;315(16):1784-6.
- Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016;387(10016):341-348.

