Diabetic Painful Neuropathy – Managing the Advanced Disease
Dr Muriel Bellizzi
Abstract
Diabetes is a complex chronic illness that can be associated with numerous complications. Amongst these is damage to the nervous system leading to different types of neuropathy. Pain can be a blessing in diabetic patients as painless neuropathy can lead to ulcerations. But on the other hand, having excessive pain can be debilitating by impacting the patients’ quality of life. The high prevalence of neuropathy amongst diabetic patients warrants extreme care when undergoing the history and examination of such patients to rule out any diabetic peripheral neuropathic pain. When pain is present, it is essential to reduce it to improve the physical functioning, reduce psychological distress and ultimately improve the quality of life.
Introduction
Diabetes Mellitus (DM) represents a major health and economic burden as well as a global epidemic. In 2019, a worldwide estimate of 463 million adults between the age of 20 and 79 years of age suffered from diabetes. The International Diabetes Federation gave an estimated global prevalence rise to 10.4% by 2040 compared to 8.8% in 2015.1 In 1981 a national WHO study held in Malta confirmed an exponential rise in diabetes in Malta. This was mainly attributed to the ageing population.2A higher prevalence of diabetes was also confirmed when compared to neighbouring countries.3
The commonest cause of neuropathy is diabetes.4 Diabetic neuropathy is one of the complications that diabetic patients commonly suffer. It is a disabling and debilitating condition. The pathophysiology of this type of neuropathy is very complex and unclear but is thought to be mainly attributed to the microvascular injury to the vasa nervorum resulting in different types of diabetic neuropathy because of damage to the nervous system. The commonest type of neuropathy is the distal symmetric polyneuropathy. Also relatively common is injury to different fibres causing mononeuropathy multiplex, diabetic amyotrophy and autonomic neuropathy.
Diabetic Peripheral Neuropathic Pain (DPNP)
Approximately 30-50% of patients with diabetes will eventually develop neuropathy.6 Of these, 2-21% will complain of painful neuropathy. DPNP is highly underreported by patients and under-treated by doctors. This pain is frequently accompanied by depression, anxiety, and sleep disorders. The risk factors for developing DPNP include type 2 diabetes mellitus, women and patients of south Asian origin.
History and examination
Diabetes UK’s footcare pathway for diabetics suggests an annual review of the foot. During the review, one should enquire about any pain which may be present, and if present, investigated (table 1).7
| Annual foot review: (Foot examination with shoes and socks removed) |
| · Test foot sensation using 10g monofilament or vibration |
| · Palpate foot pulses |
| · Inspect for any deformity |
| · Inspect for significant callus |
| · Check for signs of ulceration |
| · Inspect footwear |
| · Ask about any pain |
Table 1: Annual foot review for patients suffering from DM.
Pain varies in intensity, with most cases having moderate pain (numerical rating score (NRS) of 4-6), but around 30% of patients with diabetic neuropathy will suffer from severe pain (NRS 7-10). It is mostly described by patients as spontaneous burning pain in the peripheral limbs (see figure 1 for distribution of pain).8
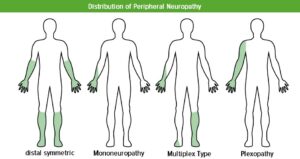
Figure: Distribution of pain
Key findings in Distal symmetrical polyneuropathy include the following:
- Numbness, tingling pain or weakness; stocking-and-glove distribution.
- Patients with diabetic neuropathy are 2-3 times more likely to fall than those with diabetes and no neuropathy.
- Patients with severe neuropathy are at risk of ulcerations and lower-extremity amputations, with 15% developing an ulcer during the course of their disease.
Tools for Detection
The tools for detection of neuropathy are quite simple and cheap. These include the 10g monofilament test and the vibration perceptive test (figure 2). The American Diabetes Association (ADA) suggests the simple test in table 2 to identify any small or large-fibre neuropathy.9
| ADA -2019 Neuropathy assessment tools: the following clinical tests may be used to assess small and large fibre function and protective sensation: |
| 1. Small-fibre function: pinprick and temperature sensation |
| 2. Large-fibre function: vibration perception and 10g monofilament |
| 3. Protective sensation: 10g monofilament |
Table 2: Identification of small or large fibre neuropathy.
|
10g Monofilament Test
|
Vibration Perception Test with 128Hz tuning fork
|
Figure 2: Tools for detection of neuropathy.
Targets of management of DPNP
Neuropathy can be prevented with tight blood glucose control and strict screening with monofilament and tuning fork. Tight glycaemic control will help decrease the incidence but does not reverse the process. Once neuropathy is diagnosed, the only treatment available to these patients consists of improving glucose control and controlling the pain with various drug groups, the commonest being the anticonvulsants and antidepressants since the process is not reversible. The use of opioids as analgesics in the long term should be avoided. This is especially important when co-prescribing opioids and gabapentinoids as the combination can increase the risk of opioid-related overdose and death.10 Managing DPNP should also focus on the improvement in other aspects which might arise because of such neuropathy including sleep, mood, functional capacity, quality of life as well as the prevention of any progression of the neuropathy and foot ulceration.
| NICE Clinical guide on the management of DPNP in non-specialist setting |
|
1. For people with painful diabetic neuropathy: a. Offer oral duloxetine as first-line treatment b. If contraindicated, offer oral amitriptyline |
|
2. For people with persistent painful diabetic neuropathy: a. If first-line treatment was with duloxetine, switch to amitriptyline, pregabalin or gabapentin , or combine with pregabalin or gabapentin b. If first-line treatment was with amitriptyline, switch to or combine with pregabalin or gabapentin |
| If satisfactory pain reduction is not achieved with second-line treatment refer the person to a specialist pain service |
Table 3: Pharmacological management of DPNP.11
NICE guidelines suggest the use of duloxetine, amitriptyline, pregabalin and gabapentin as the drugs of choice in managing DPNP (table 3,). If these are not satisfactory, the patient should be referred to the pain specialist. 50% pain improvement in DPNP trials on pregabalin and gabapentin have been registered in 47% of patients and up to 48% of patients with duloxetine. The reduction rate has not shown to be dose dependent for duloxetine, but it is for pregabalin (27% reduction with150mg vs. 47% reduction with 600mg) and gabapentin (15.9% reduction with 600mg vs. 47.6% reduction with doses 900mg-3600mg).11 Limited data is available on amitriptyline.
Other medications such as topical capsaicin, lidocaine patches, botulinum toxin and isosorbide dinitrate spray are less effective in managing DPNP.12-16
| Class | Mode of Action | Dose | Commonest adverse effects |
| TCAs (amitriptyline, nortriptyline) | Serotonin and noradrenaline reuptake inhibition, sodium channel block, NMDA receptor antagonist | 25-150mg daily | Dry mouth, dizziness, constipation, weight gain, vision problems, sexual dysfuction, sedation, prolonged QT interval |
| SNRIs (duloxetine, venlafaxine) | Serotonin and noradrenaline reuptake inhibition |
Duloxetine 60-120mg daily; Venlafaxine 150-225mg daily |
Adverse effects can be more severe than gabapentinoids such as dizziness, fatigue, nausea and insomnia |
| Gabapentinoids (gabapentin, pregabalin) | Calcium-channel blockers, reducing the release of presynaptic transmitters | Gabapentin 1200-3600mg three times daily; pregabalin 300-600mg twice daily | Dizziness, increase risk of suicide, headache, sedation, weight gain |
Table 4: Different classes of drugs used in DPNP
What can the Pain Clinic at Mater Dei Hospital offer?
Pharmacotherapy is not the only option for these DPNP patients. Neuromodulation has been suggested as last resort when other therapies have failed. Neuromodulation is a technique used to stimulate the nervous system by means of implantable devices (figure 3). This procedure is commonly used for deep brain stimulation for essential tremor and Parkinson’s disease, cochlear implants for deafness and vagal nerve stimulation for epilepsy. In chronic pain, the stimulation relates to the dorsal column in the spinal cord. A current is applied to the large diameter fibres in the dorsal spinal cord by means of two leads that enter the epidural space via an epidural needle and move towards the desired site; these are then attached to a battery similar to a pacemaker.17 The exact mechanism is still unclear, but the most plausible explanations are the following two. First, by propagating the current up the brain and stimulating the periaqueductal grey it causes a descending inhibitory pathway initiation known as orthodromic stimulation i.e. in the direction of the fibres. The second postulation is that the downwards propagation, known as antidromic stimulation against the direct of current transmission, simulates the inhibitory neurones. These inhibitory neurones will ultimately cause dampening of the stimulation coming into the spinal cord. This reduces the extent of continuous stimulation from fibres in patients with PDNP, which ultimately causes less transmission of pain.
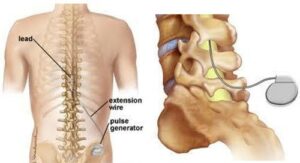
Figure 3: Neuromodulation performed at Mater Dei Hospital.18
Conclusion
DPNP is common and is both underreported by patients and undertreated by doctors. As DPNP can negatively impact patients’ quality of life, a bio-psychosocial approach to treatment is required. Prevention of neuropathy is possible if it is detected early. About half of the patients achieve a 50% improvement with current recommended treatments for DPNP. However, many patients remain poorly controlled due to inability to access adequate medication to control their pain or due to poor tolerance to the recommended medications. Referral to the pain clinic is recommended in patients with refractory pain.
References
- International Diabetes Federation. IDF Diabetes Atlas 9th Ed. Brussels, Belgium; International Diabetes Federation,
- Katona G, Aganovic I, Vuskan V, et al. National Diabetes Programme in Malta: Phase I and II Final Report. Geneva: World Health Organization; 1983.
- Cuschieri S. The diabetes epidemic in Malta. SEEJPH Published online: 19 February 2020.
- Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11(6):521-34.
- Johannsen L, Smith T, Havsager AM, et al. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J Clin Neuromuscul Dis 2001;3(2):47–52
- Maser RE, Steenkiste AR, Dorman JS, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38(11):1456–61.
- Diabetes UK. https://www.diabetes.org.uk/guide-to-diabetes/complications/feet/taking-care-of-your-feet
- Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29(7):1518-22.
- American Diabetes Association. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019 Jan;42(Suppl 1):S124-S138.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med. 2017;14(10):e1002396.
- Neuropathic pain in adults: pharmacological management in non-specialist settings. Clinical guideline (CG173). http://guidance.nice.org.uk/CG173
- Ormseth MJ, Scholz BA, Boomershine CS. Duloxetine in the management of diabetic peripheral neuropathic pain. Patient Prefer Adherence 2011;5:343-56.
- Tandan R, Lewis GA, Krusinski PB, et al. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care 1992;15(1):8–14.
- Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Capsaicin Study Group. Diabetes Care 1992;15(2):159-65.
- Yuen KC, Baker NR, Rayman G. Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: a double-blind placebo-controlled cross-over study. Diabetes Care 2002;25(10):1699–703.
- Agrawal RP, Choudhary R, Sharma P, et al. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pract 2007;77(2):161–7.
- Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur J Pain. 2019 Apr;23(4):652-659.
- https://www.sportspainmanagementnyc.com/spinal-cord-stimulation-therapy