Ultrasound Assessment of right upper quadrant abdominal pain – Part 3
Dr Pierre Vassallo
Parts 1 and 2 of this article discussed the importance of having an efficient diagnostic algorithm for right upper quadrant (RUQ) abdominal pain, since it is one of the most common presenting complaints to any clinic or emergency department. While acute cholecystitis is the most frequent cause of RUQ pain, more than a third of cases are due to other conditions. The said articles also showed that abdominal ultrasound is the imaging modality of choice because it is easily accessible, rapid, cost-effective, and safe since it involves no ionising radiation or potentially nephrotoxic intravenous contrast agent.1 Abdominal US visualises multiple upper abdominal organ systems that could be the source of the patient’s pain.
Parts 1 and 2 of this article described the US findings related to the biliary, hepatic, pancreatic and gastrointestinal causes of RUQ pain, while this final Part 3 will deal with renal, adrenal, thoracic and vascular causes.
Renal Causes of RUQ Pain
Upper urinary tract obstruction by stones is a common cause of right upper abdominal pain; classically this is described as pain radiating from the loin to the groin on the side of obstruction. Renal obstruction is readily diagnosed on ultrasound by the presence of hydronephrosis (Fig 1a). A search for the obstructing stone often finds it located at the junction between the renal pelvis and the ureter or at the point of entry of the ureter into the bladder (Fig 1b). Stones located in between these levels are rarely visible on ultrasound and are best localised and measured on non-contrast CT. Location and size of the stone are important parameters since they determine whether a stone may pass spontaneously or require surgical intervention.
Infection in the right upper urinary tract may present with acute RUQ pain; more typically, it presents with flank pain, fever, rigors and dysuria. The most common form of upper urinary tract infection is bacterial pyelonephritis, which is most frequently seen in women aged 15 to 40 years. Bacterial pyelonephritis consists of infection in the ureter and pelvicalyceal system that extends into the renal parenchyma and is usually due to ascending infection from the bladder. This may occur even in the absence of vesico-ureteric reflux and is thought to be due to functional obstruction resulting from bacterial endotoxins that inhibit ureteric peristalsis. While US cannot distinguish a sterile upper urinary tract obstruction from a dilated ureter and pelvicalyceal system due to pyelonephritis, debris within the distended pelvicalyceal system may be seen in the latter case (Fig 2). The nephritis aspect of pyelonephritis is often occult on US but may appear as a segmental area of increased echogenicity (Fig 3a). On contrast-enhanced CT, nephritis presents as an area of hypoperfusion (Fig 3b). Pyonephrosis and renal abscess formation (Fig 4) are potential complications of pyelonephritis that may occur in the absence of timely percutaneous drainage of the infected collecting system and antibiotic therapy.
Spontaneous haemorrhage into a renal cyst or neoplasm may present with RUQ pain. The most common tumours to present with spontaneous haemorrhage are angiomyolipomas particularly if they are over 4cm in diameter (Fig 5). Less commonly, haemorrhage may be seen in renal adenocarcinomas. Occasionally, haemorrhage may obscure the causative lesion on imaging. Repeat imaging with US, CT and/or MRI after resolution of the haemorrhage may be required to identify the cause.
Adrenal Causes of RUQ Pain
The adrenal gland is rarely a source of RUQ pain. The main adrenal condition that leads to pain is intraglandular haemorrhage. When symptoms are present, they may include flank pain and low grade fever. Symptoms of adrenal insufficiency would only occur with bilateral adrenal involvement and when more than 90% of adrenal tissue is destroyed. Spontaneous adrenal haemorrhage is seen in conditions of stress (surgery, severe burns and sepsis), pregnancy, bleeding diatheses and with anticoagulant therapy.2 Benign and malignant tumours of the adrenal gland may also undergo haemorrhage, which may require serial imaging follow-up to identify the causative lesion. Only large adrenal haematomas are visible on ultrasound (Fig 6). Smaller ones are only visible on CT or MRI. Intraglandular haemorrhage initially appears as a solid mass, which later shows central fluid consistency and may proceed to develop rim calcification. Once intraglandular haemorrhage has resolved, a residual cyst or dystrophic calcifications may persist within the gland.
Thoracic causes of RUQ pain
Any condition that results in right basal pleuritis can result in RUQ pain. These conditions include a right lower lobe pneumonia, pleural effusion, pulmonary infarction (pulmonary embolism) and pulmonary/chest wall neoplasms. An abscess originating in the lung from pneumonia or in the liver (e.g. amoebic abscess) may cross through the diaphragm. If peripherally located near the chest wall, these conditions may be detected by ultrasound (Fig 7). However, CT is the most reliable tool for evaluating the organs on either side of the diaphragm.
Chest wall tumours are mostly metastatic lesions originating from other organs. However, primary chest wall tumours such as lymphomas or sarcomas are also seen in clinical practice. While CT and MRI may help identify the primary lesion in the case of metastatic disease, US is very useful for confirming the presence of a chest wall mass and for guiding biopsy particularly in case of an unknown primary tumour.
Vascular causes for RUQ pain
Thrombosis of the hepatic veins, portal veins and hepatic arteries may present with RUQ pain. These three entities can be distinguished from one another with US.
Hepatic artery thrombosis is the most common complication of liver transplantation. It may however also occur due to tumour infiltration, hypercoagulability disorders, vasculitis (e.g. systemic lupus erythematosus), eclampsia and as a complication of surgery. Due to its dual blood supply (hepatic artery and portal vein), the liver is relatively resistant to hepatic infarction or ischaemic hepatitis. An altered hepatic arterial flow pattern on spectral and colour Doppler US may be noted with altered, diminished or absent flow (Fig 8). In the transplanted liver, hepatic arterial occlusion has more severe consequences; contrast-enhanced US or CT angiography (Fig 9) are required to assess the extent of arterial occlusion and of transplant infarction.
Portal vein thrombosis may be asymptomatic; however, it may present with RUQ or diffuse abdominal pain particularly if associated with mesenteric vein thrombosis. Portal vein thrombosis may be due to benign causes or due to an infiltrating tumour from adjacent organs. The distinction is important as the treatments and prognoses for both conditions differ. Benign (or bland) portal venous thrombosis may be due to a hypercoagulability state, inflammatory conditions including vasculitis, inflammation of adjacent organs (pancreas, bowel, biliary tree), use of the oral contraceptive pill and pregnancy. Superior mesenteric vein thrombosis has also been observed in high intensity athletes wearing tight running clothing and experiencing dehydrated states. The pain experienced in portal vein thrombosis is mainly due to bowel ischaemia, which may progress to bowel infarction. The thrombus in the portal vein is seen as a filling defect on US (Fig 10) and diminished monophasic flow with normal flow direction (hepatopetal) may be evident on spectral Doppler US in the case of incomplete portal venous occlusion.
Malignant portal vein thrombosis is most commonly due to invading hepatocellular carcinoma, which is itself more common in the presence of hepatic cirrhosis. Tumor thrombus in the portal vein may also arise from pancreatic carcinoma, cholangiocarcinoma and metastatic disease. One possibility to distinguish bland from tumour thrombus in the portal vein is to identify its continuity with the adjacent tumour in the latter case. Another feature is the presence of vascularity within the thrombus in the case of tumour thrombus (Fig 11), while bland thrombus is avascular. Cavernous transformation of the porta hepatis is a consequence of portal vein thrombosis; it is usually evident on colour Doppler US with absent portal vein flow and numerous surrounding tortuous collateral vessels (Fig 12).
Hepatic vein thrombosis is more commonly primary (75% of cases) due to a venous condition such as thrombosis, stenosis or web formation; it is classified as secondary (25% of cases) when it is due to extrinsic tumour compression. The clinical picture is usually of Budd Chiari Syndrome (BCS), which consists of the clinical triad of RUQ pain, hepatomegaly and ascites. US is the imaging modality of choice showing hepatomegaly (particularly caudate lobe enlargement), a coarsened liver texture, absent hepatic veins with collateral hepatic veins, a compressed inferior vena cava, splenomegaly and ascites (Fig 13). Absence or reversed flow may be noted in the hepatic veins, and identification of collaterals draining into the subcapsular and intercostal veins are highly specific signs of BCS.
Conclusion
This three-part article clearly presents the complexity of the right upper abdominal quadrant due to the presence of numerous organs each providing its own spectrum of disease. US is a rapid and efficient exam that not only guides further diagnosis and management but often alone provides detailed and specific diagnoses. To harvest the full potential of US in the diagnosis of RUQ pain one requires a sonographer with considerable experience as well as a detailed knowledge of the possible aetiologies and sonographic findings related to all the disease entities described above. A subtle or even equivocal US finding can prompt further evaluation with more complex imaging modalities such as CT or MR and could expedite the diagnostic process.
References
- American College of Radiology. ACR Appropriateness Criteria: right upper quadrant pain. https://acsearch.acr.org/docs/69474/Narrative/. Published 1996. Updated 2013. Last review date: 2013. Accessed January 26, 2018.
- Jordan E, Poder L, Courtier J, et al. Imaging of nontraumatic adrenal hemorrhage. AJR Am J Roentgenol 2012;199(1):W91–W98.
Figure Legends
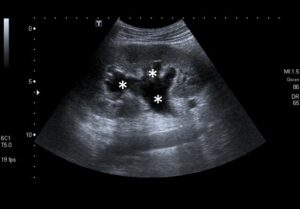
Figure 1a: Longitudinal US scan of the kidney showing a dilated PC system (*).
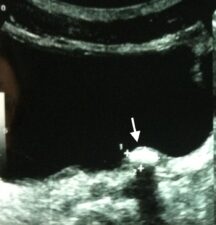
Figure 1b: Transverse US scan of the bladder showing a stone located at the ostium of the right ureter (arrow).
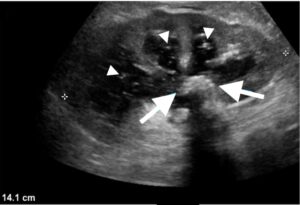
Figure 2: Longitudinal US scan of the kidney showing a dilated PC system that contains a stone (arrows) and debris (arrowheads).
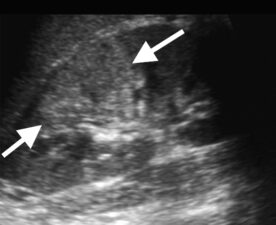
Figure 3a: Longitudinal US scan of the kidney showing at segmental area of hyperechogenicity (arrows) that correlates with focal nephritis.
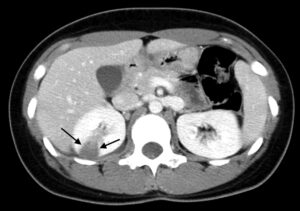
Figure 3b: Contrast-enhanced CT scan showing a segmental area of hypoperfusion (arrows).
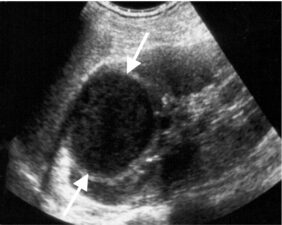
Figure 4: Longitudinal US scan through the upper pole of the kidney showing a well-defined hypoechoic lesion (arrows) that represents a renal abscess.
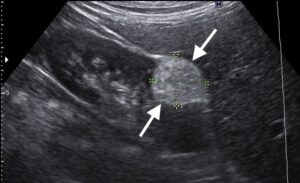
Figure 5: Longitudinal US scan through the kidney showing a well-defined hyperechoic mass (arrows) in the anterior portion of the lower renal pole, a typical appearance of an angiomyolipoma.
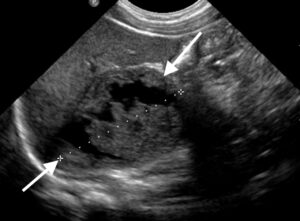
Figure 6: Longitudinal US scan through an enlarged heterogeneous right adrenal gland (arrows); the altered size and texture are due to spontaneous intraglandular haemorrhage that occurred during pregnancy.
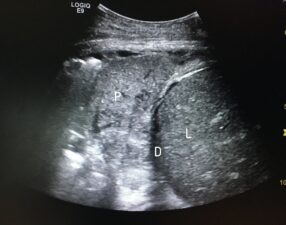
Figure 7: Intercostal US scan showing consolidation in the right lung base due to pneumonia (P) and the adjacent diaphragm (D) and liver (L).
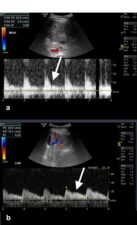
Figure 8a. Spectral Doppler US through the main hepatic artery shows low peak systolic flow velocity and a high resistive index proximal to the occlusion. b. Spectral Doppler US distal to the occlusion in the left main hepatic artery shows diminished flow and a relatively flat waveform.
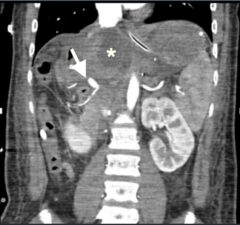
Figure 9: CT angiogram showing main hepatic arterial occlusion (arrow) in a transplanted liver with areas of liver infarction (*).

Figure 10: Longitudinal US scan through the main portal vein showing echogenic thrombus filling the said vein (arrow).
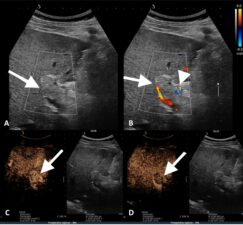
Figure 11a. Longitudinal US scan through the portal vein showing tumour thrombus (arrow). b. Colour Doppler US scan through the thrombosed portal vein (arrow) showing vascularity within the thrombus (arrowhead). c. Contrast-enhanced US showing early (arterial phase) enhancement within the tumour thrombus (arrow). d. Delayed-phase contrast-enhanced US showing washout of contrast within the thrombus (arrow).
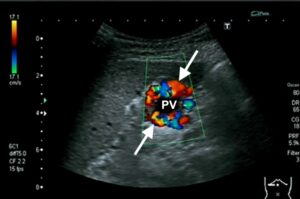
Figure 12: US scan through the portal vein showing no flow centrally within the portal vein (pv) and numerous tortuous collateral vessels around the portal vein (arrows).
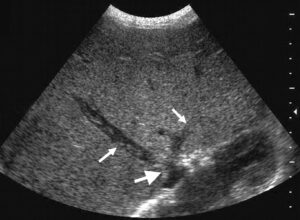
Figure 13: Transverse US scan through the liver showing thrombus in the hepatic veins (small arrows) and stenosis of the inferior vena cava (larger arrow).

