Imaging Ovarian Tumours – Part II
Dr Pierre Vassallo
Introduction
In the first article of this series we outlined the large spectrum of ovarian tumours which are grossly divided into four groups: epithelial surface cell tumours, germ cell tumours, sex cord/stromal tumours and metastases.1
A general introduction of the subtypes of each tumour group was presented and the most common tumour group, the serous epithelial tumours, was discussed in more detail.
This second article in the series will discuss mucinous epithelial tumours, other less common epithelial tumour (endometrial and clear cell carcinomas) and epithelial-stromal tumours (Brenner tumours, adenofibroma and cystadenofibromas).
Mucinous Epithelial Tumours of the Ovary
Mucinous epithelial tumours of the ovary are second most common type of ovarian tumour after serous epithelial tumours. They account for 10-15% of all ovarian tumours.2
Mucinous tumours are classified into three subgroups: mucinous cystadenomas, borderline mucinous tumours, and mucinous cystadenocarcinomas. Unlike serous epithelial tumours, in which the high-grade serous cystadenocarcinoma appears to be a distinct entity from the serous cystadenoma, borderline serous epithelial tumour and low-grade serous cystadenocarcinoma, mucinous tumours appear to arise from the same continuum of tumours that range from the benign to the overtly malignant subtype.
Most mucinous tumours encountered are of the benign or borderline type; they tend to affect a younger age group and have an excellent prognosis.3 In contrast, mucinous cystadenocarcinomas tend to occur in an older age group (mean 54 years) and have the poorest prognosis of all ovarian tumours.4
Mucinous tumours are more likely to be unilateral, multilocular, confined to the ovary and large on presentation. They often present with abdominal discomfort and distension.
In keeping with the above, mucinous epithelial tumours present on imaging studies as large, unilateral, multilocular lesions with cyst contents that show variation in signal intensity, attenuation, and echogenicity (MR, CT, US) depending on the viscosity of the mucinous content of the cysts.
Papillary projections are rare while calcifications are common in mucinous tumours; both features are in contrast to serous epithelial tumours.
Distinguishing between benign and borderline mucinous tumours may be very difficult. However the presence of numerous cystic spaces, high T1 and low T2 signal of cyst content, mural nodules and cyst walls, which are thicker than 5mm are suggestive of a borderline lesion5 (Figure 1).
Benign and borderline mucinous tumours may contain solid components, which are often composed of fibrous tissue. An abundance of fibrous stroma has led to the term mucinous cystadenofibroma.6
Mucinous cystadenocarcinomas are usually larger (>10cm) and they contain numerus small cystic spaces and large solid components7 (Figure 2).
Mucinous adenocarcinomas and, to a lesser extent, borderline mucinous tumours may exhibit seromucinous glandular cells that are similar to those seen in the gastrointestinal tract and in the cervical canal. In such situations, it is important to distinguish between a primary mucinous tumour of the ovary and a metastatic lesion to the ovary from a gastrointestinal primary neoplasm. Since primary mucinous neoplasms are usually large (>10cm) and solitary, a radiologic suggestion of small and bilateral mucinous tumours should prompt search for a gastrointestinal primary lesion.8
Endometroid and Clear Cell Carcinomas
Endometroid and clear cell ovarian tumours are almost always invasive and malignant. This is in contrast to serous and mucinous ovarian neoplasms. They are usually detected early and present in the 5th decade. High grade endometroid carcinomas are often histologically indistinguishable from high-grade serous cystadenocarcinomas (HGSCs), which raises the question as to whether the former is really a distinct entity.
Clear cell carcinomas are associated with thromboembolic disease and paraneoplastic hypercalcaemia.
Both endometroid and clear cell carcinomas are frequently associated with endometriosis and also with Lynch syndrome, which often presents with a synchronous endometrial carcinoma.9
Endometroid and clear cell carcinomas have similar imaging features to other ovarian epithelial tumours with cystic and solid components; however they tend to have more solid components than serous and mucinous ovarian tumours (Figure 3). They may arise in an endometriotic cyst, where a mural enhancing nodule in the cyst wall may be the only indication of their presence, which should raise suspicion particularly in a woman aged 45 years or older.
Brenner Tumours
Brenner tumours are rare epithelial-stromal tumours that are mostly benign and may co-exist with other epithelial neoplasms. They frequently contain amorphous calcifications and show low blood flow on colour Doppler ultrasound. Due to a large fibrous component, they show low signal on T2-w images (Figure 4). When lying adjacent to a cystic structure, the latter usually represents a co-existing epithelial lesion (usually mucinous cystadenoma). Cysts and areas of high T2 signal within Brenner tumours are rare and indicative of a malignant variety.
Adenofibroma and Cystadenofibroma
These are rare epithelial/stromal tumours that are almost always benign and often detected incidentally. Most are of the serous subtype but may be associated with other epithelial types. Their fibrous stroma may have hormonal activity.
They may show both solid and cystic components, absent colour Doppler flow, low T2 signal due to dense fibrous stromal components and minimal contrast enhancement (Figure 5). High T2 signal and stronger contrast enhancement are signs of a rare malignant variety called cystadenocarcinofibroma.
Conclusion
The above article concludes the subject of epithelial and epithelial/stromal ovarian tumours. The next and final article on the subject of ovarian tumours will discuss germ cell tumours and sex chord stromal tumours and metastases to the ovary.
Figure Legends
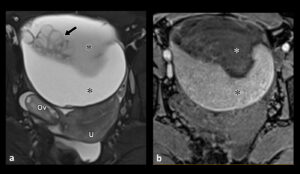
Figure 1. Borderline Mucinous Tumour: (a) T2-w transverse MRI scan showing a left ovarian tumour containing mucous of different signal intensities (*). Note the cluster of cysts (arrow) contained with the lesion. The normal right ovary (Ov) and uterus (U) are also shown. (b) T1-w transverse non-enhanced MRI scan shows low T1 signal in the mucous that showed high T2 signal in (a).
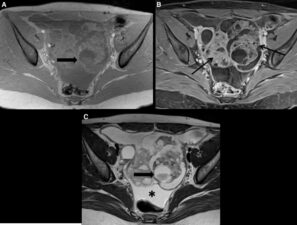
Figure 2. Mucinous Cystadenocarcinoma: T1-w fat saturated non-enhanced (a) and contrast enhanced (b) images and T2-w image through the pelvis showing a large complex mass containing numerous cystic spaces and marked enhancement of the intervening solid components (thin arrows). A fluid-fluid level (large arrow) indicates different compositions of mucous with low T2 signal (c) and intermediate T1 signal (a).
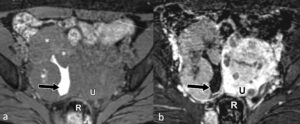
Figure 3. Endometroid Carcinoma: Pre-contrast (a) and contrast-enhanced (b) T1-w scans through the pelvis showing a right ovarian mass composed of mostly solid enhancing components (*) and a small cystic space with high T1 signal indicated haemorrhagic fluid. A fibroid uterus (U) and the rectum are also seen.
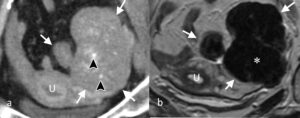
Figure 4. Brenner Tumour: (a) CT scan through a left ovarian mass (white arrows) that contains calcifications (arrowheads). (b) T2-w MRI scan showing large low signal areas in the mass (*) indicative of fibrous tissue. (U – uterus).
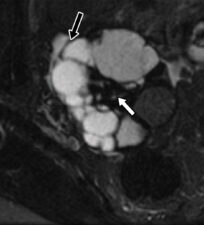
Figure 5. Cystadenofibroma: T2-w MRI scan showing a complex cystic mass with hypointense fibrous components (black arrow) and thick low signal septal walls (white arrows).
References
- Taylor EC, Irshaid L, Mathur M. Multimodality Imaging Approach to Ovarian Neoplasms with Pathologic Correlation. Radiographics 2021;41(1):289-315.
- Marko J, Marko KI, Pachigolla SL, et al. Mucinous Neoplasms of the Ovary: Radiologic-Pathologic Correlation Radiographics 2019;39(4):982-997.
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol 2018;80:11-27.
- Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst 2019;111(1):60-68.
- Zhao SH, Qiang JW, Zhang GF, et al. MRI in differentiating ovarian borderline from benign mucinous cystadenoma: pathological correlation. J Magn Reson Imaging 2014;39(1):162-6.
- Mills AM, Shanes ED. Mucinous Ovarian Tumors. Surg Pathol Clin 2019;12(2):565-585.
- Ferreira CR, Carvalho JP, Soares FA, et al. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. Int J Gynecol Cancer 2008;18(1):59-65.
- Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol 2003;27(3):281-92.
- Fadare O, Parkash V. Pathology of Endometrioid and Clear Cell Carcinoma of the Ovary. Surg Pathol Clin 2019;12(2):529-564.

