Cervical Cancer: The Importance of Screening and Vaccination
Dr Sharona Falzon, Dr Mandy Collict, Prof. Isabel Saliba
Keywords: Cervical Cancer, Cervical Screening Programme, HPV (Human Papilloma Virus), HPV Vaccination
Abstract
Cervical cancer is the fourth most common cancer in women worldwide and it is the most common cancer in women aged 15 to 44 years. The national cervical screening programme was introduced in Malta in 2016, aiming to increase the detection rate of pre-malignant, low- and high-grade cervical disease. The Human Papilloma Virus (HPV) is the main cause of cervical cancer and with the HPV vaccine as part of the national immunization programme, we have a strong and useful weapon in the prevention of infection with this virus.
Cervical cancer
Cervical cancer originates at the transformation zone which surrounds the opening of the cervix leading into the endocervical canal (Figure 1). The ectocervix is a layer of squamous cells on the outer surface of the cervix whilst the endocervix consists of mucus producing glandular columnar cells making up the inner surface of the cervix. If cells from the ectocervix become cancerous it will lead to a squamous cell carcinoma, which is the most common type of cervical cancer. The glandular cells of the endocervix can also become cancerous, leading to an adenocarcinoma of the cervix.1 Less commonly, cervical cancers have features of both squamous cell carcinomas and adenocarcinomas. These are called adenosquamous carcinomas or mixed carcinomas.
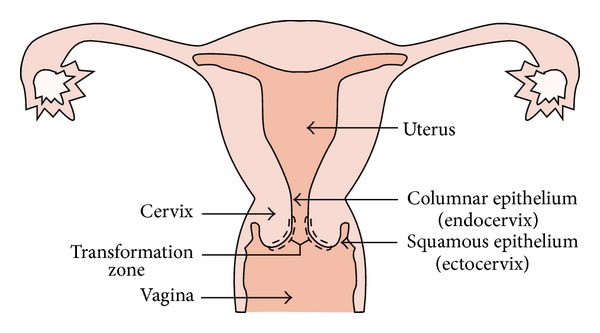
Figure 1: Female Reproductive System2
Cervical cancer can be detected at a pre-malignant stage due to presence of dyskaryotic atypical cells within the squamous epithelium, better known as cervical intra-epithelial neoplasia (CIN) or cervical dysplasia. CIN is a histological diagnosis and depends on the extent of invasion. CIN I refers to mild dysplasia whereby atypical cells are found only in the lower 1/3 of the epithelium. CIN II refers to moderate dysplasia with atypical cells found in the lower 2/3 of epithelium. CIN III refers to carcinoma in situ, where atypical cells occupy the full thickness of the epithelium. In CIN III the cells are similar to malignant lesions but show no invasion beyond the epithelial basement membrane.3
Cervical cancer is the commonest type of cancer in women under the age of 35 years in developed countries. It is the fourth most common cancer in women worldwide and the second most common in low- and middle-income countries.4 It has an overall 65% five-year survival and is thus a major cause of morbidity and mortality from cancer.3 Cervical cancer isn’t always obvious and may not cause any symptoms until it’s reached an advanced stage. In most cases, vaginal bleeding is the first noticeable symptom. The most common symptoms of cervical cancer include intermenstrual, postcoital or postmenopausal bleeding. Cervical cancer may also be associated with vaginal discharge, dyspareunia or pelvic pain.5
Risk Factors
The major cause of cervical cancers is Human Papilloma Virus (HPV). There are over 130 strains of HPV with the majority being harmless, causing genital warts, and some others being associated with cervical cancer.3 HPV types 16 and 18 are responsible for around 70% cervical cancer cases.6 HPV18 is mainly a risk factor for the development of adenocarcinoma whereas HPV16 is associated with both squamous cell and adenocarcinoma. Other high-risk strains include HPV type 13, 31, 33.3 For most people, the immune system clears the HPV infection spontaneously within 2 years. In cases of persistent infection, especially with a high-risk type of HPV there is a higher risk of developing CIN leading to cervical cancer.6 The risk is even higher in women who have other sexually transmitted infections alongside of HPV, especially Herpes Simplex Type 2 virus.
Other risk factors include:
- Immunodeficiency
- Smoking
- Taking the oral contraceptive pill for more than five years
- High parity6
- Becoming sexually active at a younger age (especially <18 years).7
Prevention and Early Detection
Regular cervical screening can detect CIN changes and is a simple and effective way to pick up cervical cell changes. The cervical screening test was independently invented in the 1920s by Drs Georgios Papanikolaou and Aurel Babeș and named after Papanikolaou. The German virologist, Prof. Harald zur Hausen discovered the link between HPV and cervical cancer and was awarded the Nobel Prize for Physiology and Medicine in 2008 for this discovery.
Conventional Papanicolaou (PAP) smears can give false results due to inadequate sampling and slide preparation, and errors in laboratory detection and interpretation. On the other hand liquid-based cervical cytology has been developed to improve the diagnostic reliability of the test. Cervical cells are rinsed in a liquid based medium, which prevents false results due to blood or other potentially obscuring materials. Liquid-based cytology also allows for additional testing for HPV from the same sample in high risk cases.8
An abnormal cervical screening test result does not mean cancer. Most abnormal results are due to signs of HPV, the presence of treatable precancerous cells (CIN), or both, rather than cancer itself.6
With an abnormal smear result, the next investigation in line would be colposcopy +/- biopsy. This procedure also allows for staining with acetic acid and Lugol’s iodine which help detect abnormal cells, as well as biopsies of the cervix. CIN I commonly regresses spontaneously but if left untreated CIN II and III will usually develop into cervical cancer over a period of 10 years, therefore detection and treatment are crucial. CIN can be treated by doing a large loop excision of the transformation zone using cutting diathermy under local anaesthetic. Established cervical cancer requires further investigation to determine FIGO classification and histology for staging. Treatment ranges from cone biopsy to radical hysterectomy +/- radiotherapy and chemotherapy.3
Practising safer sex by using barrier methods like condoms will reduce the risk of getting HPV and passing it on but is not 100% effective. There are now vaccines available to prevent HPV infection. HPV vaccination provides safe, effective, and lasting protection against the HPV infections that most commonly cause cancer. These vaccines do not protect against all kinds of HPV, therefore one still needs to take part in cervical screening, even if vaccinated.6 The vaccines available are Gardasil 9 (recombinant HPV nonavalent vaccine, Types 6, 11, 16, 18, 31, 33, 45, 52, and 58) and Cervarix (recombinant HPV bivalent vaccine, Types 16 and 18). Both vaccines are recommended from the age of 9 years.9
Between the ages of 9 and 14, the vaccine is given in 2 doses spaced 6 months apart. After the age of 14, the course is of 3 doses, with the first 2 doses given 1 month apart and the third dose given 5 months after the second dose. In Malta, HPV vaccination is part of the National Immunisation Schedule and is given free of charge to girls born from 2000 onwards on reaching their 12th birthday.9 Previously girls were vaccinated with Cervarix but in recent months Gardasil 9 started being administered. The earlier the vaccination, the better the response, since increasing age is linked to more persistent infection. Previous HPV infection does not provide immunity to future infection and it is not yet known whether the vaccine offers lifelong protection. Males have the potential to be HPV carriers, therefore it may be advisable to offer them the HPV vaccine as well. In keeping with this, since September 2019 the UK has extended its vaccination programme to include twelve-year-old boys.6 This is a key factor in stopping spread of vaccine-preventable diseases and hopefully Malta will take on board free male HPV vaccination through the National Immunisation Schedule sooner rather than later.
Malta’s Screening Programme
Women aged twenty-five to forty-nine years are offered screening every three years and those aged fifty to sixty-four years are offered screening every five years.10 The national screening programme has been in place since 2016 but many females were aware of the importance of cervical screening long before that. Unfortunately, the national screening programme is very poorly attended with less than 30% attendance rate. It is important to note that this is not representative of the females who are actually getting screened since several opt to get screened privately. Ideally the data from the screening programme and private gynaecologists is merged to get a clear idea of how many people are aware of cervical cancer and the need for screening. Until then, medical professionals are encouraged to promote screening to help combat cervical cancer as it is a preventable disease.
Acknowledgements
I would like to express my appreciation to Ms Lina Sultana from the National Cervical Screening Programme who kindly offered her input.
References
- Cancer Research UK. Cervical Cancer. 2 August 2017. [Online] Available at: https://www.cancerresearchuk.org/about-cancer/cervical-cancer
- Bengtsson E, Malm P. Screening for cervical cancer using automated analysis of PAP-smears. Comput Math Methods Med 2014;2014:842037.
- Impey L, Child T. Obstetrics and Gynaecology, 5th Ed. Wiley-Blackwell Publishing Company; 2017.
- Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri [published correction appears in Int J Gynaecol Obstet. 2019 Nov;147(2):279-280]. Int J Gynaecol Obstet. 2019;145(1):129-135.
- Mwaka AD, Orach CG, Were EM, et al. Awareness of cervical cancer risk factors and symptoms: cross-sectional community survey in post-conflict northern Uganda. Health Expect 2016;19(4):854-867.
- Poudel K, Sumi N. Analyzing Awareness on Risk Factors, Barriers and Prevention of Cervical Cancer among Pairs of Nepali High School Students and Their Mothers. Int J Environ Res Public Health 2019;16(22):4382.
- Nkfusai NC, Cumber SN, Anchang-Kimbi JK, et al. Assessment of the current state of knowledge and risk factors of cervical cancer among women in the Buea Health District, Cameroon. Pan Afr Med J 2019;33:38.
- Chrysostomou AC, Stylianou DC, Constantinidou A, et al. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018;10(12):729.
- Government of Malta. Cervical Screening. 2020. [Online] Available at: https://deputyprimeminister.gov.mt/en/phc/nbs/Pages/Screening-Programmes/Cervical-Screening.aspx

