Venous Thromboembolism
Dr Pierre Vassallo
Definition
Venous thromboembolism (VTE) is an umbrella term that includes deep venous thrombosis (DVT) and pulmonary embolism (PE).
Introduction
VTE is a significant cause of morbidity and mortality. It is the third most common cardiovascular condition worldwide following myocardial infarction and stroke.1 The 30-day mortality rates for patients with DVT and PE are 3% and 31% respectively.2
VTE may be isolated (no identifiable cause) or may be secondary to a considerable number of conditions that lead to a hypercoagulable state. The clinical history and an understanding of the risk factors for VTE may point to an underlying cause.
Conversely, knowledge of the predisposing factors may help detect subtle VTE and its complications on ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) studies.3
Multimodality imaging (US, CT and MRI) allows early detection of VTE and helps to identify its cause and any associated complications. It is also valuable to monitor the effects of treatment.
Causes
Virchow’s triad describes the three broad categories of factors, which, in isolation or combined, contribute to an increased risk for venous thrombosis (Fig 1).2 Venous stasis, damage to the vascular endothelium and conditions leading to hypercoagulable states all increase the likelihood of thrombosis.
Many conditions influence more than one of these mechanisms to increase the risk of thrombosis. The link between hypercoagulable states and malignancy has been well established for most types of malignancy. Some malignancies invade vessels and hence cause endothelial damage; in this group of tumours, the most notable are renal cell cancer, hepatocellular carcinoma, adrenal cortical cancer and non-hyperfunctioning pancreatic neuroendocrine tumours. Tumours may also lead to vascular compression and venous stasis, further increasing the likelihood of thrombosis.
However, the main mechanism that causes VTE in patients suffering from cancer occurs through a stimulation of an inflammatory reaction, which in turn upregulates the coagulation cascade while downregulating the anticoagulative pathways.2
Infections and major trauma can also increase the risk of VTE provoked by inflammatory responses that influence the coagulative/anticoagulative pathways.2
Venous stasis may be the result of prolonged immobilisation and extrinsic vascular compression, such as by enlarged lymph nodes.4 This can cause direct endothelial damage and hypoxia that can stimulate aggregation of platelets and leukocytes.5
Some genetic conditions such as factor V Leiden mutation or deficiencies in protein S, protein C or antithrombin alter distribution of clotting factors in the blood thereby inducing hypercoagulable states (thrombophilia).6
Additional risk factors for VTE include major surgery/trauma, orthopaedic injury, oral contraceptive medication, obesity and older age.3
Detection of VTE
Ultrasound
Ultrasound is primarily used for detection of peripheral venous thrombosis, where acute thrombus can be visualised directly as hypoechoic tissue expanding the vein, absent compressibility of the vein by the examining ultrasound probe, and abnormal or absent blood flow on Doppler imaging (Fig 2).
The above technique can be used to evaluate the deep lower limb veins, which include the common, superficial and deep femoral and popliteal veins as well as the greater and lesser saphenous veins. The technique is not reliable for detecting thrombosis in deep veins below the mid-calf. In those patients with symptoms that are suspicious for DVT and who have a normal compression ultrasound, it is recommended to repeat compression ultrasound within one week to detect proximal progression of an occult distal thrombus.
While superficial vein thrombosis (e.g. in the greater and lesser saphenous veins) is considered less likely to be a source of emboli, it can extend into the deep veins and ultimately pose the same risk as DVT.
Doppler spectral waveforms are more useful than colour Doppler imaging to assess altered blood flow patterns; changes in these blood flow patterns may indicate venous thrombosis proximal or distal to the examining probe (Fig 3). This is particularly useful when assessing veins that are not accessible to compression (Fig 4).
The subclavian vein shows variation in diameter with respiration (Fig 5) frequently collapsing fully during inspiration. Absence of variation in diameter of the subclavian vein indicates proximal venous occlusion due to thrombus (e.g. with indwelling central venous lines) or extrinsic compression such as may occur with a superior mediastinal mass.
Spectral Doppler evaluation of the common and superficial femoral veins during manual calf compression shows flow augmentation (Fig 6). Absence of this flow augmentation is suggestive of thrombosis in those deep veins located between the examining probe and the compressing hand.
Acute thrombus is often hypoechoic on greyscale US and cannot be distinguished from flowing blood in the vascular lumen (Fig 4c). Organised thrombus is echogenic and usually attached to the vessel wall (Fig 7).
CT and MRI
Thrombus cannot be distinguished from flowing blood within the vessel lumen on non-contrast enhanced CT. Contrast enhancement of the blood pool is required to visualise thrombus; this appears as a hypoattenuating filling defect within the vessel lumen (Fig 8).
CT is particularly useful in deep and central veins, which are not easily accessible to ultrasound compression. It is also useful for identifying the cause (Fig 9) and for distinguishing tumour thrombus from bland thrombus. Bland thrombus differs from tumour thrombus because the latter shows contrast enhancement while the former does not. Tumour thrombus usually exhibits continuity with the primary tumour and shows enhancement patterns like those of the primary tumour.
On MRI scans, thrombus can be identified without the use of intravenous contrast material; with flow sensitive (bright blood) sequences, thrombus appears as a dark intravascular filling defect within bright flowing blood (Fig 10).
While like CT, contrast-enhanced MRI can distinguish enhancing tumour thrombus from non-enhancing bland thrombus, diffusion weighted imaging (DWI) may be used to avoid contrast material injection. Tumour thrombus shows higher signal and a lower apparent diffusion coefficient (ADC) on DWI than bland thrombus.7
Identification of the presence and extent of tumour thrombus is crucial for accurate staging, for treatment planning and for assessing prognosis.8
Conclusion
In 1865, the French physician Armand Trousseau reported that migratory thrombophlebitis may indicate the presence of occult malignancy. Today, Trousseau’s syndrome refers to unexplained thrombotic events, which may precede or occur in parallel with occult visceral malignancy. VTE is seven times more common in patients with known malignancy that in those without. Detection of VTE should therefore prompt a search for underlying malignancy. Conversely, when a malignant lesion is detected on imaging, a search for secondary VTE needs to be considered.
Multimodality imaging allows detection and accurate assessment of extent of VTE. While clinical history may provide a clue to the underlying cause of VTE, multimodality imaging is most useful for detecting co-existing malignant disease, which is often occult at the time of presentation.
Figure Legends
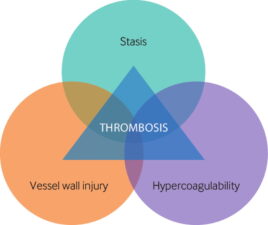
Figure 1. Virchow’s Triad outlines the mechanisms that predispose to venous thrombosis.
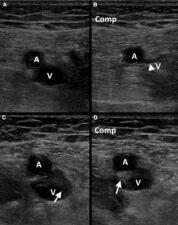
Figure 2. a and b. Normal popliteal vein without (a) and with (b) compression: Note that the vein (V) collapses (arrowhead in b) on compression by the ultrasound probe. c and d. Thrombosed of the popliteal vein without (c) and with (d) compression: Vein (V) does not collapse on compression. The thrombus (arrow in c and d) is easily seen. A is the popliteal artery.
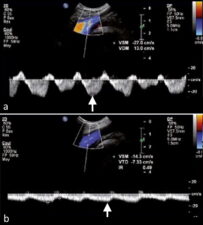
Figure 3. a. Longitudinal triplex doppler scan of the left common femoral vein shows normal phasic flow that fluctuates with respiration. b. On the longitudinal triplex doppler scan of the right common femoral vein, marked dampening of the flow phasicity is noted due to common iliac vein thrombosis. A triplex doppler scan is a scanning mode that delivers a greyscale image with colour and spectral doppler images simultaneously in real-time.
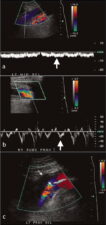
Figure 4. Spectral flow phasicity is markedly dampened in the left mid subclavian vein (a) compared to the right (b). (c) Scan of the proximal left subclavian vein shows fresh thrombus as an area of absent flow (arrows) on colour doppler imaging. This patient developed left brachiocephalic/subclavian vein thrombosis after aortic valve replacement surgery.
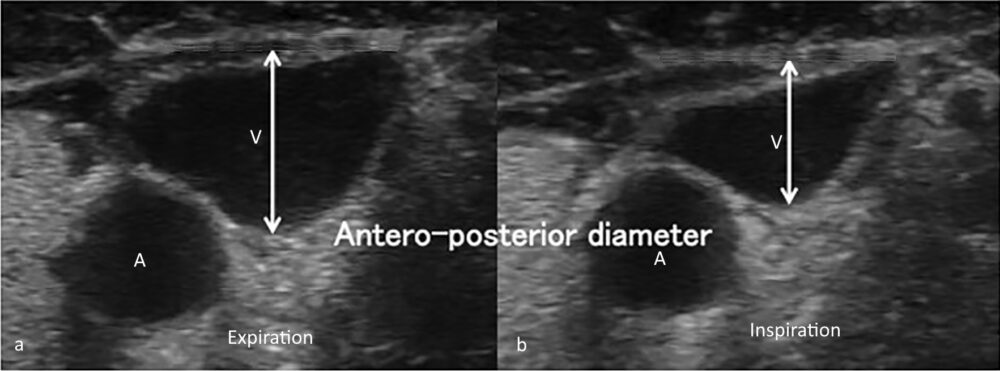
Figure 5. Left subclavian shows normal fluctuation in diameter during expiration (a) and inspiration (b). Absence of such fluctuation in diameter would indicate brachiocephalic vein obstruction either by thrombus or through external compression.
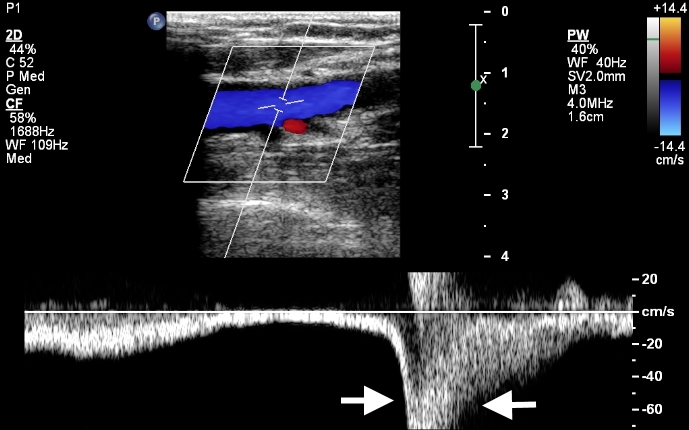
Figure 6. Augmentation of flow through calf compression: spectral doppler image obtained during calf compression (arrows) shows augmentation of flow. This phenomenon helps to exclude thrombosis in deep veins more distal to the examining probe.
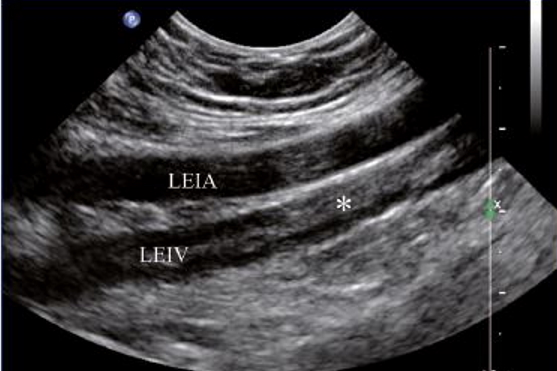
Figure 7. Left external iliac vein (LEIV) contains organised echogenic thrombus (*). (LEIA: left external iliac artery).
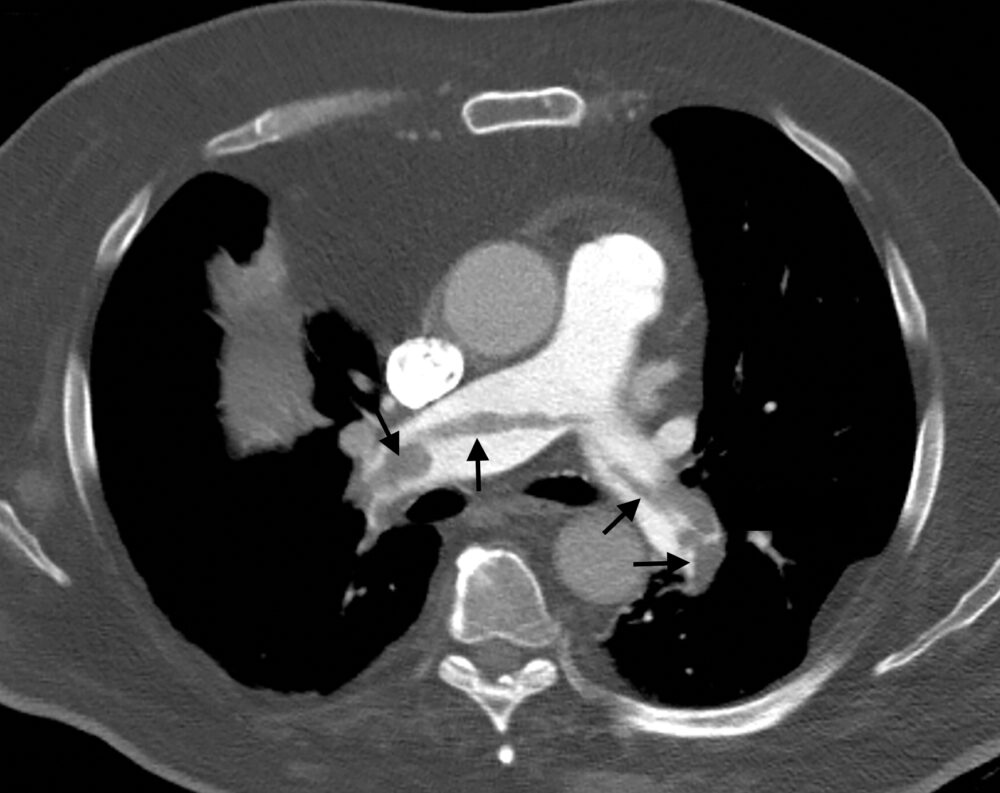
Figure 8. Hypoattenuating filling defects (arrows) in both main pulmonary arteries on contrast enhanced CT represent pulmonary emboli, which are thrombi that have migrated from the deep veins of the lower extremities. .
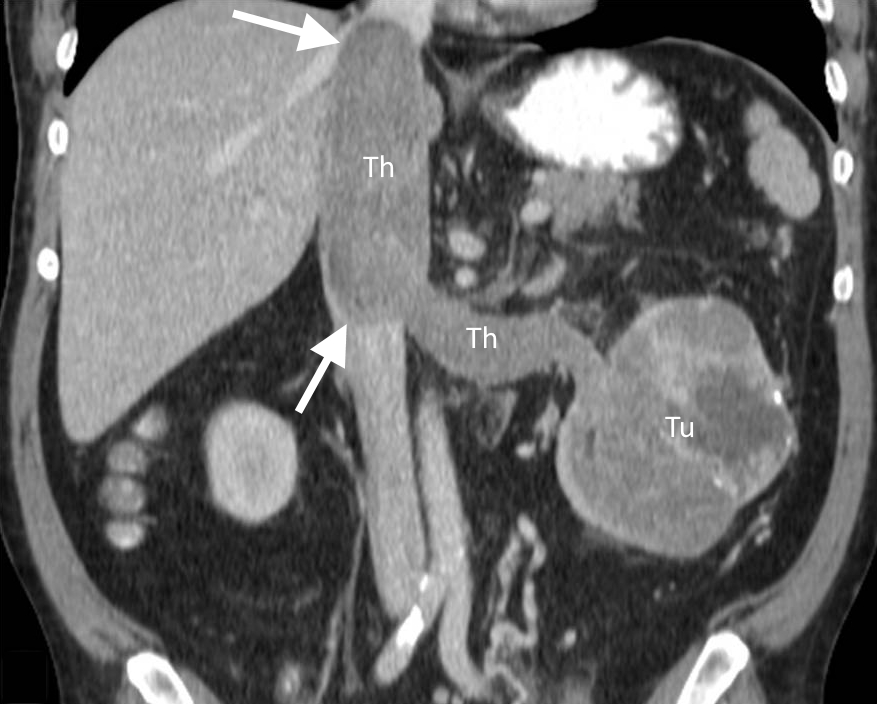
Figure 9. CT showing a left renal cell carcinoma (Tu) with tumour thrombus (Th) extending into the left renal vein and inferior vena cava.
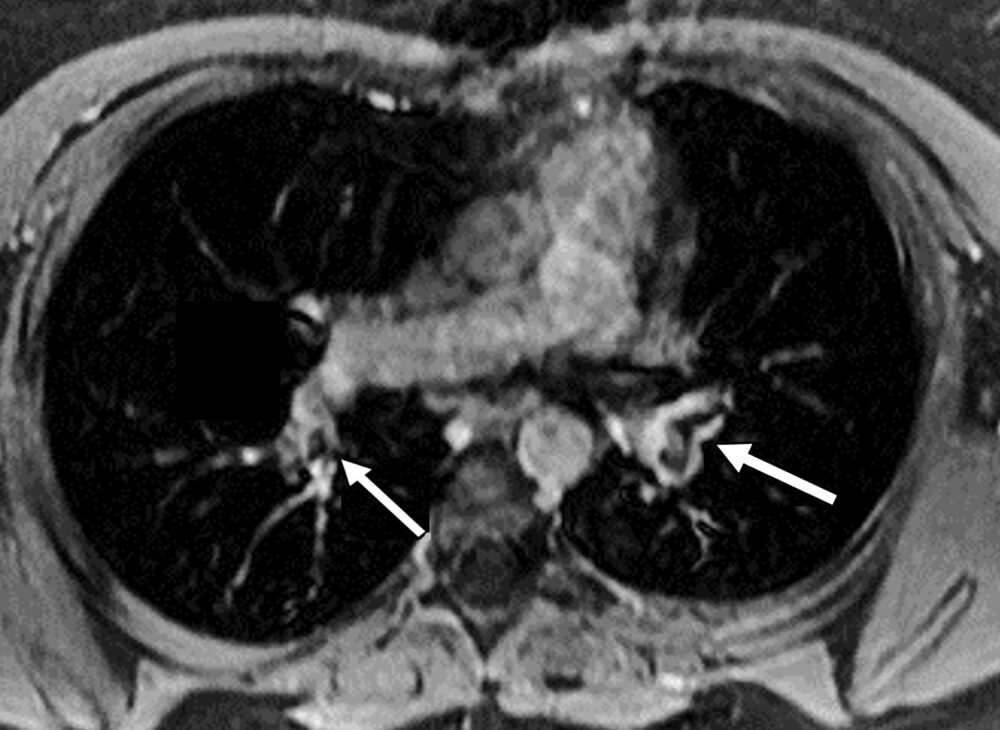
Figure 10. MRI bright blood sequence showing filling defects (arrows) in the main branches of the pulmonary arteries in a patient on oral contraceptives.
References
1. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med 2013;126(9):832.e13-21.
2. Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost 2015;113(6):1176-83.
3. Olson MC, Lubner MG, Menias CO, et al. Venous Thrombosis and Hypercoagulability in the Abdomen and Pelvis: Causes and Imaging Findings. Radiographics 2020;40(3):875-894.
4. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ 2002;325(7369):887-90.
5. López JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res 2009;123 Suppl 4:S30-4.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med2011; 13(1):1–16.
7. Catalano OA, Choy G, Zhu A, et al. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology 2010;254(1):154-62.
8. Ikushima S, Ono R, Fukuda K, Sakayori M, Awano N, Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol 2016;46(3):204-8.

