Imaging Ovarian Tumours Part III
DR PIERRE VASSALLO
INTRODUCTION
In the first article of this three-part series, we outlined the large spectrum of ovarian tumours which are grossly divided into four groups: epithelial surface cell tumours, germ cell tumours, sex cord/stromal tumours and metastases.1
A general introduction of the subtypes of each tumour group was presented and the most common tumour group, the serous epithelial tumours, was discussed in more detail. The second article discussed mucinous epithelial tumours, other less common epithelial tumours (endometrial and clear cell carcinomas) and epithelial-stromal tumours (Brenner tumours, adenofibroma and cystadenofibromas).
This third and final article in the series will deal with germ cell tumours and sex chordstromal tumours and metastases to the ovary.
GERM CELL TUMOURS
Ovarian Germ Cell Tumours (OGCTs) are true primary ovarian neoplasms since they arise in embryonic germ cells that originate within the ovary. Since germ cells are the precursor cells to all embryonic tissues including the placenta, OGCTs constitute many different tumour types. They include teratomas (mature, immature and monodermal types), dysgerminomas, yolk sac tumours (previously called endodermal sinus tumours), embryonal carcinomas, polyembryomas and choriocarcinomas.
OGCTs are the second most common type of ovarian tumor after epithelial neoplasms. They are usually benign (95%) and tend to affect the younger age group. Some tumour types may be distinguished clinically by the presence of specific tumour markers.
The mature teratoma (also known as dermoid) is the most common OGCT. It occurs at a mean age of 32yrs and is usually asymptomatic. Large tumours may cause pain and may undergo torsion. These tumours are benign with malignant transformation occurring rarely (2% of cases) and in an older age group (>40yrs).2 Other rare complications include tumour rupture, infection, autoimmune haemolytic anaemia and paraneoplastic encephalitis.3
Mature teratomas characteristically contain sebaceous material, fat, hair, teeth, bone or cartilage. Fat components are hyperechoic on US (Fig. 1a) and hypodense on CT (Fig. 1b). Teeth, bones and calcified cartilage are best seen on CT (Fig. 1c). On MR imaging, fat components show high signal on T1-weighted images and low signal on T2-weighted images with low signal on fat-suppressed images (Fig. 1d and e). A fat/fluid level may occasional be noted within a mature teratoma and is usually detected on all three imaging modalities. Fat suppressed T1-weighted images are crucial for distinguishing fat signal from haemorrhage; the latter remains hyperintense on fat-saturated T1-weighted images.
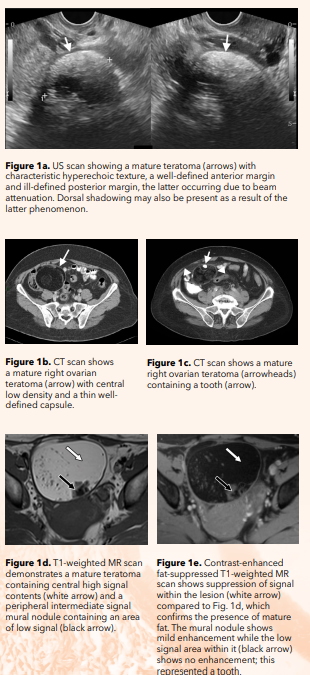
By definition, mature teratomas should contain at least two of the three germ cell layers (ectoderm, mesoderm and endoderm).
Immature teratomas comprise the malignant counterpart to mature teratomas. They are the most common malignant germ cell tumour4 and are seen in younger women. They are often large and therefore palpable at the time of presentation.
Immature teratomas are larger that mature teratomas at the time of imaging. They appear more heterogeneous, with more solid contrast-enhancing components, containing irregular calcifications rather than coarse and tooth-like structures, and containing fluid rather than fat (Fig. 2a).
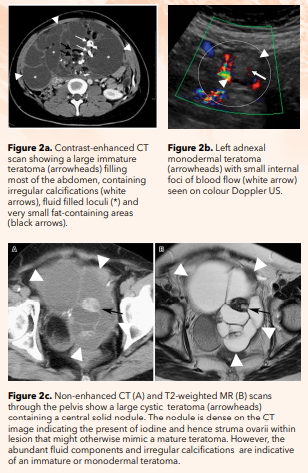
Foci of haemorrhage are common within immature teratomas. The tumour grade is based on the presence and amount of neuroepithelium. The amount of yolk sac tumour that may be present within immature teratomas is the source of α-fetoprotein (AFP) and is useful for monitoring response to treatment and for detecting tumour recurrence.5
Monodermal teratomas are rare and consist predominantly of a histologically mature cell type, most commonly thyroid tissue (in the case of struma ovarii). However, they can also contain carcinoid and neural tumours. Struma ovarii may lead to hyperthyroidism and rarely, malignant transformation may occur (mostly to papillary carcinoma).6
On imaging, monodermal teratomas usually appear similar to mature teratomas but with solid components that either display blood flow detectible on colour Doppler US (Fig. 2b) or show avid contrast enhancement on CT and MR imaging. Thus, if flow is detected on colour doppler imaging or solid contrast avid components are noted on CT or MR within an otherwise classic appearing mature teratoma, the possibility of struma ovarii must be considered.
In addition, high iodine content that is common in thyroid tissue and hence within struma ovarii leads to high density on CT (Fig. 2c).
Dysgerminomas are the ovarian counterparts of testicular seminomas. They are the second most common OGCT and manifest in adolescents or young adults. They show rapid growth leading to abdominal distension and pain. Non-specific elevation of lactate dehydrogenase (LDH) and alkaline phosphatase may occur. Elevation of β-human chorionic gonadotrophin (β-hCG) may be caused by syncytiotrophoblasts (placental precursor cells) present within the tumour.7
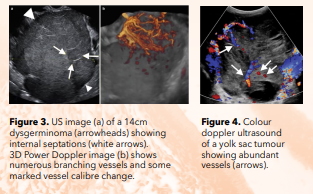
At imaging, dysgerminomas are large (>10cm), predominantly solid, unilateral, with intervening fibrovascular septa, solid enhancing components and increased vascularity on colour Doppler imaging (Fig. 3). Speckled calcifications may also be present. In comparison with other predominantly solid tumours, such as Brenner tumours and fibromas, dysgerminomas contain less fibrous tissue and hence show higher T2 signal on MR imaging.
Yolk sac tumours are rare and aggressive tumours that present in young females (mean age 19yrs) and have poor prognosis. They grow rapidly and are associated with increased AFP levels like dysgerminomas. They are usually large at the time of diagnosis and contain cystic and solid components, the latter showing high vascularity on colour Doppler US (Fig. 4) and enhancement on CT and MR imaging. Haemorrhage is common within this tumour.
Other malignant OGCTs including choriocarcinoma, embryonal carcinoma, polyembryoma and mixed germ cell tumours are all rare and very aggressive tumours. Choriocarcinoma may be gestational and non-gestational; the latter are seen in children and young women who present with precocious puberty and exhibit high β-hCG levels. These tumours are large, predominantly solid and highly vascular which may contain areas of haemorrhage similar to yolk sac tumours.7 Embryonal carcinomas, polyembryomas and mixed germ cell tumours may also cause increasing levels of β-hCG, but in these tumours we also see elevations in AFP and LDH.
SEX CORD-STROMAL TUMOURS
Primitive sex cord cells (granulosa and Sertoli cells) and stromal cells (fibroblasts, thecal cells and Leydig cells) surround the oocytes in the ovary and are involved in hormone production (such as oestrogens, androgens and corticoids). Thus, tumours originating from this cell group are likely to be associated with excess oestrogen or androgen production.
Ovarian sex cord-stroma tumours are uncommon and are more often benign than malignant. They manifest early mainly due to increased hormone production, are usually small and confined to the ovary at the time of detection. Some rare subtypes may be aggressive.8
On imaging, these tumours are mostly solid with the exception of granulosa cell tumours.
Granulosa cell tumours have a variable appearance on imaging and may mimic malignant tumours presenting as large solid or mixed solid/cystic lesions with areas of haemorrhage. Due to oestrogen effect, endometrial thickening may be evident on US and MR imaging.
Extraovarian spread is rare but may occur and may present as late recurrence with peritoneal seeding and retroperitoneal deposits.9
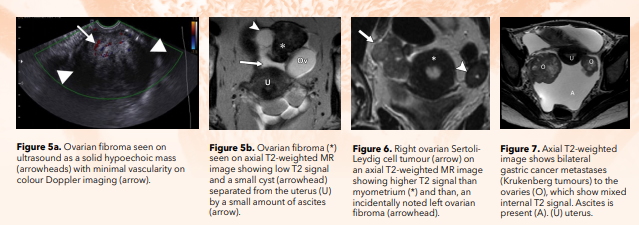
Fibromas, thecomas and fibrotechomas occur mainly in middle-aged women. Meigs syndrome (pleural effusion and ascites associated with an ovarian mass) may occur with these tumours, particularly fibromas. On US, these present as hypoechoic masses with no flow on colour Doppler imaging (Fig. 5). They may resemble a pediculated fibroid. On MR imaging, these tumours tend to have low T1 and T2 signal due to their fibrous component. However cystic degeneration and oedema may be present in larger tumours (>6cm) and in those undergoing torsion. Contrast enhancement in these lesions is usually evident on delayed imaging.
Sertoli/Leydig cell tumours are rare, mixed sex cord[1]stromal tumours that manifest mainly in young women with virilisation or amenorrhoea, vaginal bleeding from the oestrogenic effects and abdominal pain.10 These tumours appear mostly as solid masses with some cystic components and some internal colour Doppler flow and marked contrast enhancement on CT and MR imaging (Fig. 6).
Collision Tumours are neoplasms that contain more than one histologic subtype, such as a mucinous neoplasm with a germ cell tumour. This further complicates imaging diagnosis since image features of both components co-exist.
METASTASES
Metastases to the ovary occur mainly from gastrointestinal (GI) malignancies including those in the colon, appendix, stomach and pancreas. Malignancies other than GI that may spread to the ovary include breast, lung and contralateral ovarian neoplasms. Ovarian metastases may be the first signs of the presence of a distant primary tumour. In comparison with primary ovarian neoplasms, metastases are often bilateral, smaller at the time of detection, and often associated with peritoneal carcinomatosis (Fig. 7). Pseudomyxoma peritonei may be present particularly with appendiceal tumours. Ovarian metastases vary in texture on US, CT and MR imaging based on the nature of the primary tumour; gastric and breast cancer metastases tend to be solid, while appendiceal, colorectal and pancreatic metastases tend to be more cystic.11 Carcino-embryonic antigen (CEA) may be elevated in GI malignancies and increased CA-125 levels may be seen in primary serous tumours; these tumour markers may help in both diagnosis and for monitoring effectiveness of treatment.
Lymphomas of the ovary are usually secondary as part of disseminated systemic disease. This occurs within lymphocytes in the ovarian hilum. The most common lymphoma type to affect the ovary is diffuse large B-cell lymphoma.
CONCLUSION
Given the complexity and variety of ovarian cancers, one needs to maintain a clear diagnostic algorithm to ensure that detection, accurate initial staging and subsequent follow-up of patients is performed to have the best treatment outcome. Endovaginal ultrasound is the first imaging modality to evaluate a suspected ovarian mass. In case of equivocal findings or with large masses that cannot be visually encompassed by ultrasound, MR imaging should be the second imaging modality since it provides the best tissue contrast resolution and allows tumours to be clearly distinguished from adjacent organs and helps with tissue characterisation. CT is recommended for preoperative staging and follow-up of surgical or systemic treatment.
REFERENCES
- Taylor EC, Irshaid L, Mathur M. Multimodality Imaging Approach to Ovarian Neoplasms with Pathologic Correlation. Radiographics 2021;41(1):289-315.
- Comerci JT Jr, Licciardi F, Bergh PA, et al. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol 1994;84(1):22-8.
- Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA[1]receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7(12):1091-8.
- Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107(5):1075-85.
- Heifetz SA, Cushing B, Giller R, et al. Immature teratomas in children: pathologic considerations: a report from the combined Pediatric Oncology Group/Children’s Cancer Group. Am J Surg Pathol 1998;22(9):1115-24.
- Makani S, Kim W, Gaba AR. Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol Oncol 2004;94(3):835-9.
- Shaaban AM, Rezvani M, Elsayes KM, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics 2014;34(3):777-801.
- Jung SE, Rha SE, Lee JM, et al. CT and MRI findings of sex cord-stromal tumor of the ovary. AJR Am J Roentgenol 2005;185(1):207-15.
- Fotopoulou C, Savvatis K, Braicu EI, et al. Adult granulosa cell tumors of the ovary: tumor dissemination pattern at primary and recurrent situation, surgical outcome. Gynecol Oncol 2010;119(2):285-90.
- Durmus Y, Kilic C, Cakir C, et al. Sertoli-Leydig cell tumor of the ovary: Analysis of a single institution database and review of the literature. J Obstet Gynaecol Res 2019;45(7):1311-1318.
- Koyama T, Mikami Y, Saga T, et al. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imaging 2007;32(6):784-95.

