Imaging Back Pain – Part 3
Dr Pierre Vassallo
This is the third and last article about imaging back pain. The first article presented the mechanisms of discogenic back pain. This second one discussed osseous causes of back pain. This article will cover miscellaneous causes of back pain including osteoradionecrosis and insufficiency fractures, bone infarcts, traumatic fractures, facet joint disease, myofascial and paravertebral causes and spinal instability issues.1
Osteoradionecrosis and Insufficiency Fractures
Osteoradionecrosis results from exposure of bone to radiotherapy, which is used to treat bone metastases. Radiotherapy induces necrosis in bone metastases, which is followed by bony sclerosis that strengthens the bone thereby reducing risk of fractures secondary to metastatic disease.
Radiotherapy also causes a replacement of the haemopoietic elements in the bone marrow with fat. This is seen as an area of high signal intensity on T1-weighted MR scans and intermediate signal on T2-weighted MRI scans in the region that was exposed to radiotherapy (Fig 1).
Osteoradionecrosis is a severe delayed radiation-induced complication and is characterized by bone necrosis, which shows hypointensity at T1-weighted and hyperintensity at T2-weighted MR scans, with heterogeneous enhancement on administration of contrast material (Fig 2). This bone necrosis leads to insufficiency fractures.
Osteoradionecrosis results in cell necrosis, compromise of the endplates and insufficiency fractures that induce the release of inflammatory cytokines and stimulate neovascularity and neurogenesis all of which cause pain. This may be treated by surgical debridement, hyperbaric oxygen therapy and NSAIDS.
Bone Infarction
Bone infarction may be a consequence of many conditions that lead to vascular occlusion; these may include any form of vasculitis, autoimmune disease, sickle cell disease, thalassaemia and corticosteroid therapy.2 It is also seen on acute lymphocytic leukaemia and may be consequent to the malignancy itself or to treatment with corticosteroid. Bone infarction may be the first indicator of systemic disease.
Hypoxia leads to bone necrosis and invasion by macrophages, which produce pro-inflammatory cytokines that cause tissue inflammation. Sensitisation of nociceptors located in the bone marrow and periosteum to inflammatory cytokines results in pain.
Infarct bone appears as having low signal on T1-weighted images and high signal on T2-weighted images. Occasionally, high signal may be present on T1-w images due to accumulation of protein-rich fluid. Contrast enhancement is present in areas of neovascularity (Fig 3). A double line sign is pathognomonic of bone infarction but is more frequently evident in the long bones: this correlates with adjacent layers of granulation tissue (high T2 signal) and bony sclerosis (low T2 signal) at the margins of a bone infarct.
Vertebral infarction mainly involves the anterior portions of the vertebral bodies and is rarely seen in the posterior elements.
Vertebral Fractures
Vertebral fractures are frequently primary and result of trauma. However, they may also be secondary to metastatic disease, osteoporosis and osteonecrosis.
Fractures are best seen on STIR or heavily T2-weighted MR scans, where the fracture appears as linear low signal intensity and adjacent bone shows high T2 signal due to bone marrow oedema (Fig 4). Vertebral body deformity is seen in case of a burst or wedge fracture.
Neovascularity that occurs as part of the healing process causes an inflammatory response with the release of tumour necrosis factor (TNF) and interleukin 1β; both inflammatory mediators stimulate nociceptors in the bone marrow and periosteum that lead to pain. This is treated with NSAIDS and rest.
Facetogenic Pain
The facet joints are synovial joints between the superior and inferior articular processes of adjacent vertebrae. They are prone to all types of inflammatory and degenerative disease processes as other synovial joints. The articular cartilage, synovial membrane, joint capsule and the subchondral bone contain proprioceptive and nociceptive nerve endings that transmit pain stimuli to the segmental nerves.
Facet joints receive dual innervation from two adjacent segmental nerves; thus, the L4/5 facet joint receives its innervation from the descending medial branch of L3 dorsal ramus and the ascending medial branch of L4 dorsal ramus.3 Hence, pain originating from a facet joint is felt at two segmental levels, and any pain relief treatment (such as radiofrequency nerve ablation) will need to address both levels to be effective.4
Facet joint arthropathy is known to account for 30% of chronic low back pain. It is due to degenerative change that leads to hypertrophy of the facet joints. Hypertrophy of the facet joints is seen as joint enlargement with osteophytes, which may encroach on the adjacent neural foramen and cause segmental nerve root impingement. Increased fluid within the synovial joint is also seen (Fig 5). These changes in the facet joint have been shown to be induced by mechanical loading of the back and repetitive trauma. Hyperextension, rotation and lateral flexion usually increase pain. However, there is poor correlation between the extent of facet joint arthropathy and severity of back pain.
Interleukin 1β has been shown to leak out of hypertrophic facet joints and to stimulate nociceptors related to the adjacent segmental nerve.
Treatment options vary from NSAIDs to surgical decompression in case of neurological impingement. Radiofrequency ablation, cryoablation or chemical neurolysis with phenol or alcohol may be used to block pain originating within the facet joint.
Facet joint infection
Facet joint infections result from haematogenous spread of infection after bacteraemia. Facet joint infections may be accompanied by epidural (25% of cases) or paraspinal abscesses; these abscesses are seen as areas of diffusion restriction on Diffuse Weighted Imaging MR scans and show peripheral enhancement on contrast-enhanced MR scans (Fig 6).
Contrast-enhancement helps to distinguish infectious from degenerative joint disease, since the latter contrast-enhancement is minimal.5
The presence of infection results in inflammation with the release of pro-inflammatory cytokines such as TNF and Interleukin with stimulate the capsular and adjacent nociceptor and result in local pain. Treatment includes systemic antibiotics and surgical debridement is often required.
Spinal instability
Any damage to joints or soft tissues that are responsible for maintaining alignment of the spine can lead to spinal instability. Spinal instability is a state in which more movement is allowed than is under normal circumstances in a stable spine. This abnormal / increased movement results in further damage to the spinal joints. Through various mechanisms including fibrosis, bony sclerosis and new bone formation, the spine attempts to limit these abnormal movements and to restore stability. These restorative mechanisms limit vertebral slippage (spondylolisthesis) but also limit physiological movement.
The cyclical process of joint/soft tissue damage, destabilisation and re-stabilisation may continue and may result in chronic back pain. In the early stages, imaging findings may be absent. However, disk and facet joint findings become evident in the later stages.
Procedures to stabilize the spine should hypothetically prevent progressive tissue and joint damage and limit pain. However, there appear to be poor correlation between abnormal spine mobility and pain. There is better correlation between abnormal load distribution and pain.6
Myofascial and paraspinal pain
Myofascial pain has been shown to affect up to 85% of the general population at some time during their lifetime. It is caused by myofascial trigger points that are identified by means of palpation as foci of hypercontracted areas in a muscle. These result of muscle overuse or trauma, but may also result from psychologic stress.
The sustained contractile activity and cell stress occurring at myofascial trigger points induce the increased release of myokines, inflammatory cytokines, and neurotransmitters that contribute to the accentuation of the same myofascial trigger points and to myofascial pain syndrome.7
Tendinopathy of the paraspinal muscles, the clinical syndrome of pain and dysfunction in a tendon, is often a chronic condition. Symptoms of tendinopathy include localized tendon pain with loading, tenderness on palpation, and impaired function. Tendon pain has a transient intermittent nature that is closely linked to loading. Many biochemical changes occur in patients with tendinopathy. Tendon pain is likely mediated by proinflammatory cytokines that stimulate local nociceptors.
Myofascial pain and tendinopathy are rarely associated with any imaging findings and must often be identified based on clinical examination alone.
Conclusion
Low back pain presents a broad clinical and diagnostic problem for patients, physicians, and radiologists. This article and the previous two ones have attempted to demonstrate the most common causes of low back pain and to discuss diagnostic and treatment options.
Figure Legends
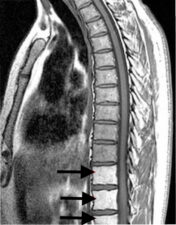
Figure 1: Sagittal MRI scan through the lower thoracic and upper lumbar spine showing high T1 signal in the lower three vertebral bodies following exposure to radiotherapy.
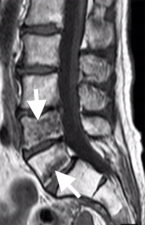
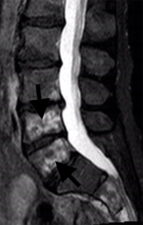
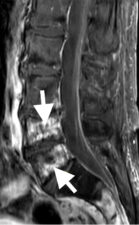
Figure 2: Sagittal scans through the lumbar spine in a patient who had radiotherapy for rectal cancer 5 years earlier show low signal intensity foci on T1-w scans (a) that represent fractures in L4 and L5 vertebral bodies. High signal foci on T2-w scans (b) correlate with areas of osteonecrosis, which enhance on contrast-enhanced T1-w scans (c).
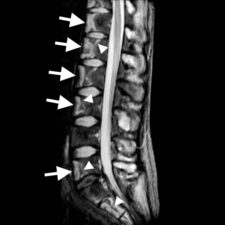
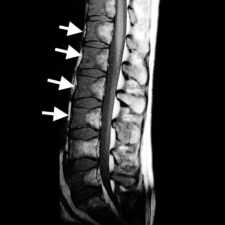
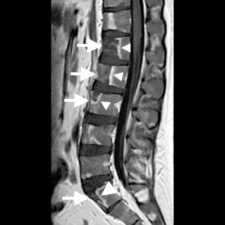
Figure 3: a. Sagittal T2-w MRI scan of the lumbar spine and sacrum showing multiple areas of high signal in the anterior portions of T11 to L4 vertebral bodies, L5 and the sacrum (arrows). Note that there is some suggestion of a double line sign in some of the bone infracts (arrowheads). b. Sagittal T1-w MRI scan of the same area shows low signal in the infarcted areas (arrows) due to replacement of fatty marrow by oedema. c. The contrast-enhanced T1-w scan shows rim enhancement (arrowheads) of the infarcts corresponding with the areas of neovascularity.
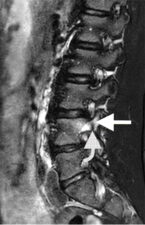
Figure 4: Sagittal STIR MR image showing a pedicular fracture as a low signal intensity line (curved arrow) with adjacent vertebral oedema that extends into the superior articular process (straight arrow).
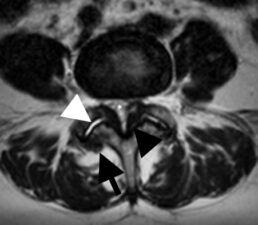
Figure 5: Transverse T2-w scan through L4/5 facet joints shows fluid in the joint cavity (white arrowhead), hypertrophy of the capsule with osteophyte formation (black arrow) and hypertrophy of the ligamenta flava (black arrowhead). Capsular hypertrophy may encroach on the neural foramen and cause segmental radicular pain. Hypertrophy of the ligamenta flava may result in spinal canal stenosis as seen in the case (*).
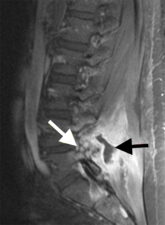
Figure 6: Sagittal contrast enhanced T1-w scan shows enhancement in L4/5 facet joint (white arrow) and a peripherally enhancing abscess cavity (black arrow) in the paraspinal tissues. There is no enhancement with the pus-containing portion of the abscess since this is avascular.
References
- Ota Y, Connolly M, Srinivasan A, et al. . Mechanisms and Origins of Spinal Pain: from Molecules to Anatomy, with Diagnostic Clues and Imaging Findings. RadioGraphics 2020;40(4):1163-1181.
- Kanthawang T, Nuttaya P, Worawit L. Acute bone infarction: a rare complication in thalassemia. Skeletal radiology 2016; 45(7):1013-1016.
- Perolat R, Kastler A, Nicot B, et al. Facet joint syndrome: from diagnosis to interventional management. Insights Imaging 2018;9(5):773-789.
- The pain source – Innervation of the lumbar facet joints.
http://thepainsource.com/innervation-of-lumbar-facet-joints/#:~:text=Because%20each%20facet%20joint%20receives%20dual%20innervation%20from,radiofrequency%20ablation%20%28neurotomy%29%20on%20two%20separate%20medial%20branches. - André V, Pot-Vaucel M, Cozic C, et al. Septic arthritis of the facet joint. Med Mal Infect 2015;45(6):215-21.
- Izzo R, Guarnieri G, Guglielmi G, et al. Biomechanics of the spine. Part II: spinal instability. Eur J Radiol 2013;82(1):127-38.
- Jafri MS. Mechanisms of Myofascial Pain. Int Sch Res Notices 2014;2014:523924.

