Breast Cancer in Young Women
Dr Pierre Vassallo
Breast cancer is the most common cancer in women affecting approximately 1 in 8 individuals worldwide in 2021;1 it is also the third most common cause of death from cancer in women.2
Breast cancer screening programs recommend yearly screening of women starting at 40 years of age unless they are considered higher-than-average risk.3 Indeed, research studies have shown that screening women starting from 40 years of age with yearly mammograms improves breast cancer survival through early detection and treatment with a resultant decrease in mortality rate of over 40%.4
Breast cancer screening in women younger than 40 years of age has so far focussed only on those who have a genetic predisposition, either through carrying the breast cancer genes or due to a strong family history. However, as in women over the age of 40 years, the overwhelming majority of breast cancers in younger women occur in individuals who have no breast cancer gene mutations (90-95%)5 or without a first-degree relative with breast cancer (89%).6
Consequently, breast cancer in younger women is mostly detected solely through clinical findings, which results in late diagnosis and poor treatment outcome. In addition, younger women tend to have more aggressive breast cancers including triple negative cancers and human epidermal growth factor receptor 2 (HER2)-rich tumours. Triple negative cancers are those cancers that test negative for oestrogen, progesterone and Human Epidermal Growth Factor 2 (HER2) receptors on immune histochemical testing.
Historically, triple negative and HER2-positive cancers have been considered more aggressive than oestrogen receptor negative (luminal) cancers. However, luminal cancers are more common than triple negative and HER2-positive breast cancers in young women. In addition, studies comparing breast cancer survival rates over the past four decades have shown that younger women with luminal cancers fare worse that those with triple negative or HER2-positive cancers.7 This is likely due to developments in breast cancer therapy, particularly adjuvant chemotherapy that have been less successful for luminal cancers. 8
Impact of Patient Age and Tumour Subtype on Breast Cancer Prognosis
Age strongly influences the likelihood of survival. The younger the age at the time of diagnosis the worse the prognosis.
The more aggressive tumour types, such as triple negative breast cancers, are more common in women <40 years old (24.9%) compared to older women (14.6%).9
Imaging Findings in Triple Negative Cancers
Ultrasound (US) findings in triple negative cancer may closely mimic those of a fibroadenoma. A subtle feature such as a more rounded appearance may be the only distinguishing US finding that would prompt further investigation (Fig 1a). Fibroadenomas tend to have a more oval shape and a horizontal orientation (Fig 1b). Due to the similar features of both lesions, core biopsy confirmation is required to guide further management. While US is a very valuable tool for the detection of and characterisation of breast nodules particularly in women <30 years of age, subtle features such as described above should prompt further imaging and biopsy. In contrast, ill-defined lesion margins are strongly suggestive of malignant disease (Fig 1c).
Mammograms characteristically show malignant lesions as having irregular lesion margins, but these are often absent in triple negative cancers (Fig 2a). Mammograms are less useful in younger women due to a higher proportion of triple negative cancers in this age group and because these women are more likely to have dense breasts.
Magnetic Resonance Imaging (MRI) has the highest sensitivity for detection of breast cancer in young women, with malignant lesions tending to show higher T2 signal due to more abundant cytoplasm, oedematous stroma, and necrosis (Fig 3a). Ring enhancement on contrast-enhanced T1-weighted images (Fig 3b) and diffusion restriction on Diffusion-Weighted Imaging (DWI) (Fig 3c) are both strong indicators of malignant disease.
Due their rapid growth, triple negative breast cancers are known to present as interval cancers on mammographic screening. Interval cancers are those cancers that grow so rapidly that they may present as sizeable tumours on a mammogram, even though no lesion was present on a previous mammogram performed 12 months earlier or less.
HER2-enriched (HER2-postive) Tumours
HER2-positive tumours comprise 25% of all breast cancers in women <40 years of age.10 HER2-rich cancers have a higher proliferative index, are more aggressive and are more likely to present with multifocal or multicentric disease and metastases.11
HER2-positive tumours are more aggressive when they are hormone-receptor (oestrogen/progesterone-receptor) negative.12
HER2-positive tumours are more likely to be associated with ductal carcinoma in situ (DCIS) and hence frequently present with microcalcifications. Digital breast tomosynthesis (or 3D mammography) is useful in assessing the extent of multifocal/multicentric disease in HER2-positive tumours by detecting foci of microcalcification (Figure 4). MRI helps further in establishing the extent of HER2-positive cancers.
Accurate mapping of these tumours is crucial to monitoring the effects of therapy particularly because HER2-positive tumours tend to respond well to cytotoxic and monoclonal antibody therapy.13
De Novo Stage IV Disease and Tumour Recurrence Rate
Women <40 years of age are four times more likely to be diagnosed with distant stage breast cancer than those aged 40-69 years14 due to lack of screening resulting in late diagnosis. This observation is further supported by the increased incidence of de novo stage IV disease over the past four decades in women under 40 years of age;15 this is likely due to both the aggressiveness of the tumour types seen in this age group and the lack of screening. A full imaging evaluation of all potential sites for extramammary metastatic disease is crucial in this age group.
Regardless of tumour subtype, women <40 years of age have a higher tumour recurrence rate after surgical treatment and a higher mortality rate than older women.16
Conclusion
Current breast cancer screening programs do not adequately address women younger than forty years of age. While some attention is given to those younger women who are breast cancer gene positive and to those who have a strong family history, none is paid to the remaining women who account for the majority of breast cancer cases in this age group.
It is important to understand the biological characteristics and imaging appearances of early breast cancer in younger women.
The higher incidence of triple negative and HER2-positive breast cancers in this age group leads to poorer outcomes. In addition, younger women with cancers considered to be less aggressive (such as luminal cancer) have shown less improvement in mortality rate that the more aggressive breast cancers in younger women over the past four decades.
Subtle findings on US may raise the level of suspicion and change management. Patients with an assumed benign lesion on US may not return for follow-up for years, potentially resulting in a late-stage cancer diagnosis. Advances in chemotherapy do not overcome the disadvantages of a late-stage diagnosis.17 Thus, any subtle, atypical findings on US should be aggressively investigated with supplementary imaging (mammography and MRI) and/or imaging-guided biopsy.
Figure Legends
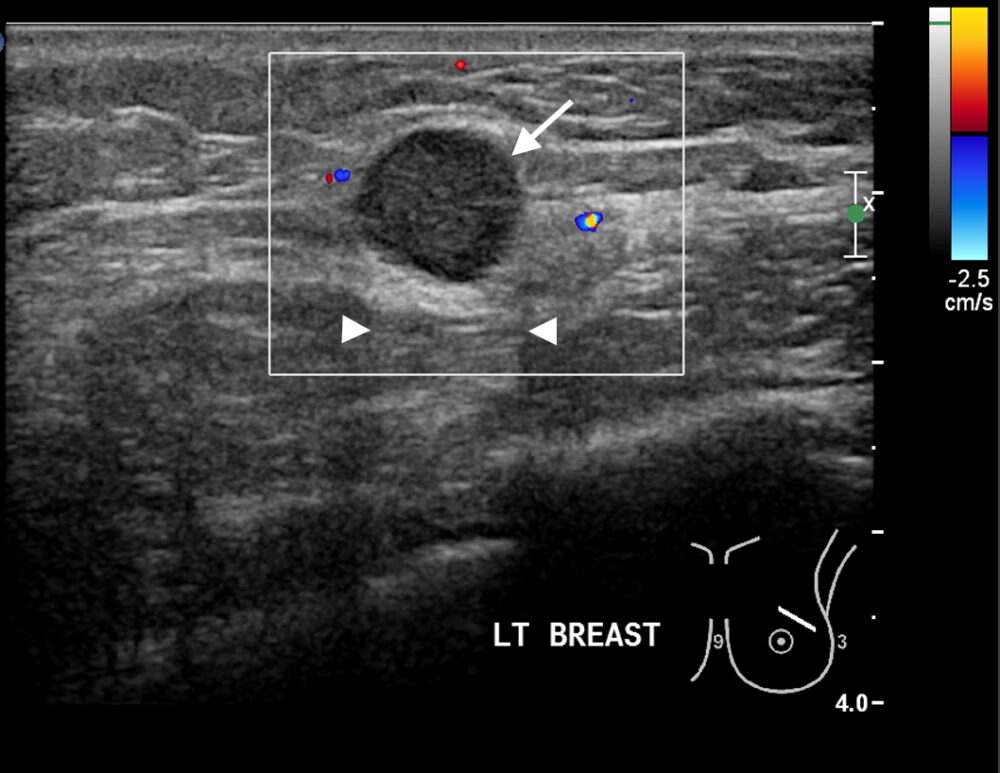
Figure 1a
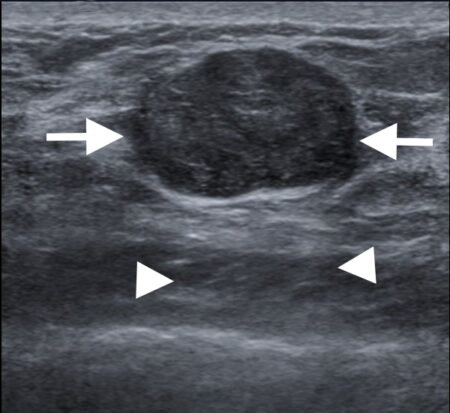
Figure 1b
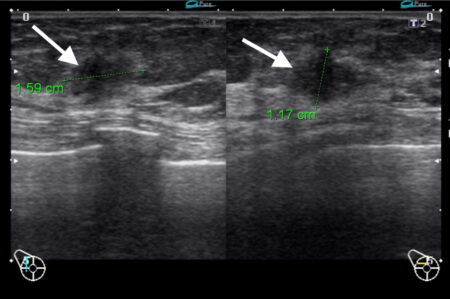
Figure 1c
Figure 1: Triple negative breast cancer vs fibroadenoma – US. a. Colour Doppler US image of a triple negative breast cancer showing a rounded well-defined hypoechoic nodule (arrow) and no appreciable internal vascularity. b. US image of a fibroadenoma showing a well-defined hypoechoic lesion (arrows) with oval shape and horizontal orientation. A central echogenic line is seen in b that is more indicative of a fibroadenoma. Dorsal enhancement, a.k.a. through transmission, is seen in both a and b (arrowheads). c. US image showing an ill-defined nodule (arrows) in the left breast that was confirmed to be invasive ductal carcinoma on US-guided biopsy (Case c courtesy of Dr Roberto Schubert, Radiopaedia.org, rID: 15840).
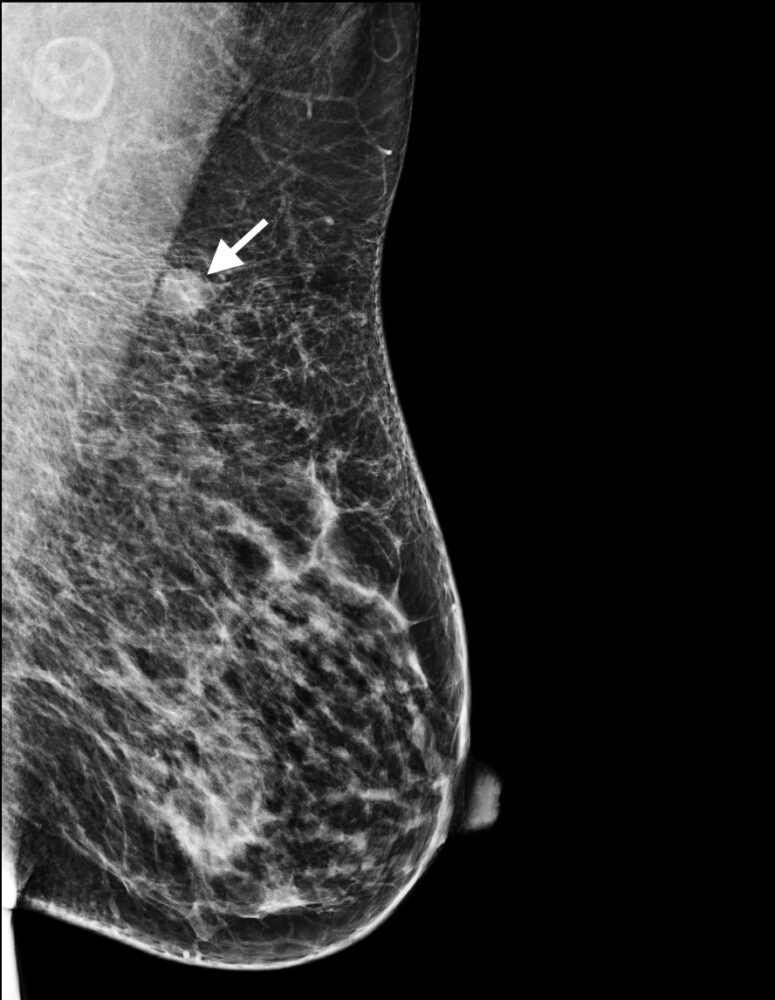
Figure 2: Triple negative breast cancer – mammogram. Left mediolateral oblique mammogram showing the same nodule (arrow) as in 1a in the superior portion of the left breast; this lesion exhibits smooth margins.
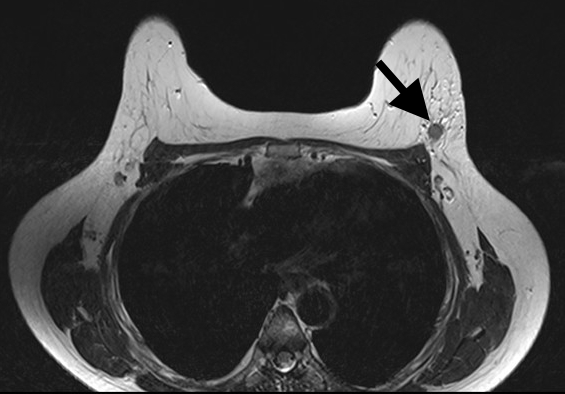
Figure 3a
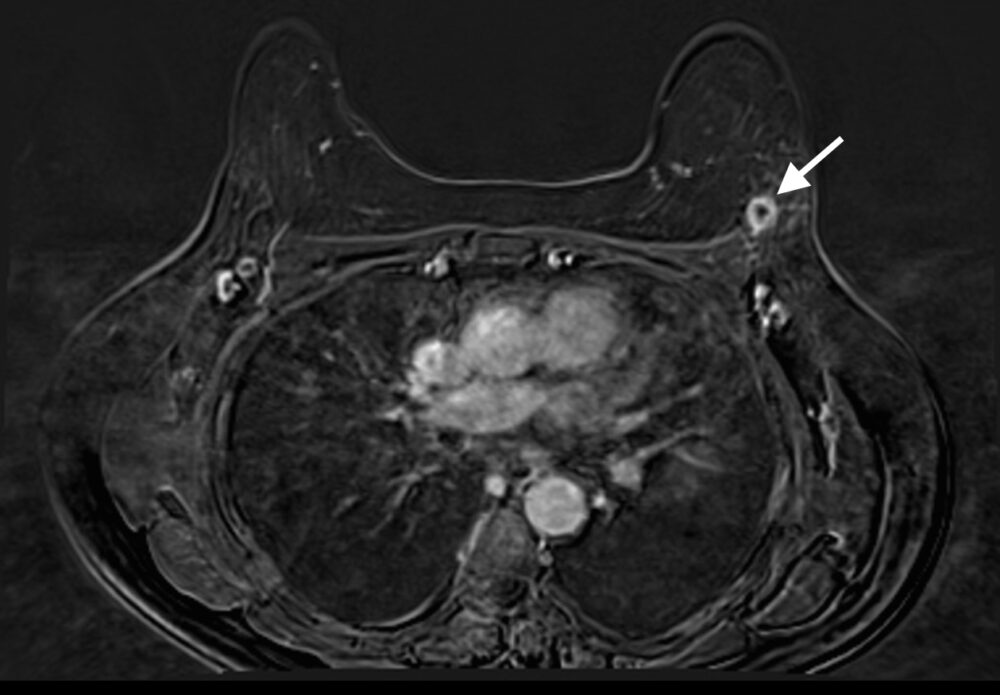
Figure 3b
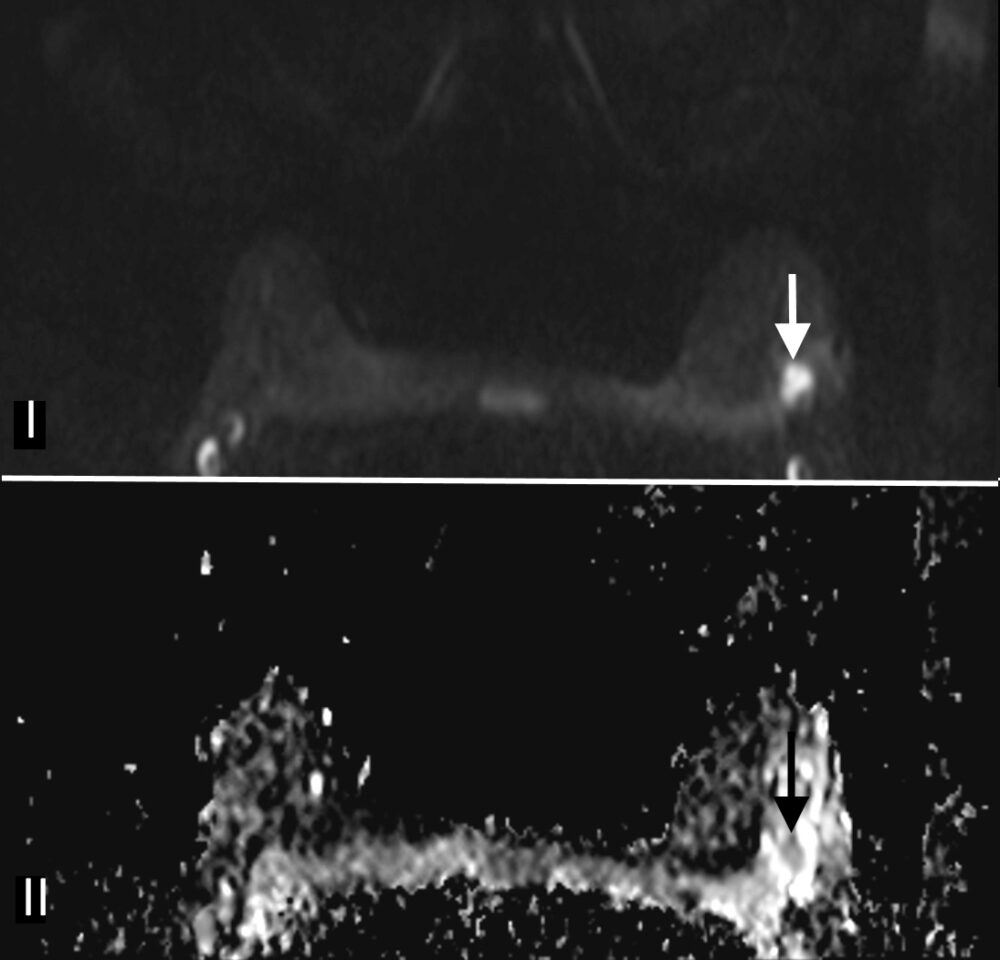
Figure 3c
Figure 3: Triple negative breast cancer – MRI. a. T2-weighted image showing a lesion in the left breast (arrow) with signal that is higher than that of adjacent pectoralis muscle. b. Contrast-enhanced fat-suppressed breast T1-weighted image shows the lesion (arrow) with rim enhancement (arrow), which helps differentiate it from a benign lesion such as a fibroadenoma. c. The same nodule (arrows) exhibits high signal on the diffusion-weighted image (I) and low signal on the ADC map (II) indicating diffusion restriction. (Case courtesy of Dr Ahmed Abdelrahman, Radiopaedia.org, rID: 78448)
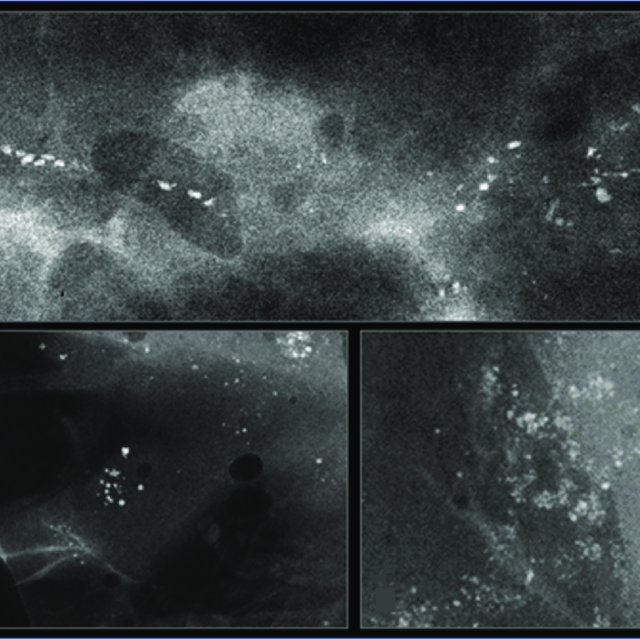
Figure 4: Mammogram showing multiple clusters of microcalcifications; these are related to DCIS that is more common in HER2-postive cancers.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. International Journal of Cancer 2021;149(4):778–89. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.33588
- Society AC. Cancer facts & figures. Cancer Facts and Statistics. 2022. p. 4.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast Cancer Screening Recommendations Inclusive of All Women at Average Risk: Update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021 Sep;18(9):1280-1288; Available from: https://www.sciencedirect.com/science/article/pii/S1546144021003835
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer 2017 Oct 1;123(19):3673-3680. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/cncr.30842@10.1002/(ISSN)1097-0142.breastcancercollection
- Claus EB, Schildkraut… JM. The genetic attributable risk of breast and ovarian cancer. Cancer 1996 Jun 1;77(11):2318-24. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1097-0142(19960601)77:11%3C2318::AID-CNCR21%3E3.0.CO;2-Z
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001 Oct 27;358(9291):1389-99. Available from: https://www.sciencedirect.com/science/article/pii/S0140673601065242
- Gao Y, Samreen N, Heller SL. Non-BRCAEarly-Onset Breast Cancer in Young Women. Radiographics. 2022 Jan-Feb;42(1):5-22. Available from: https://pubs.rsna.org/doi/abs/full/10.1148/rg.210109
- Liu YR, Jiang YZ, Yu KD, et al. Different patterns in the prognostic value of age for breast cancer-specific mortality depending on hormone receptor status: a SEER population-based analysis. Ann Surg Oncol. 2015 Apr;22(4):1102-10. Available from: https://link.springer.com/article/10.1245/s10434-014-4108-5
- Partridge AH, Hughes ME, Warner ET, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol. 2016 Sep 20;34(27):3308-14.Available from: http://blog.youngsurvival.org/wp-content/uploads/2016/10/JCO-2016-Partridge-JCO-2015-65-8013.pdf
- Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018 Feb;19(2):169-180.Available from: https://www.sciencedirect.com/science/article/pii/S1470204517308914
- Shen SC, Ueng SH, Yang CK, et al. Impact of Detection Method and Accompanying Ductal Carcinoma in Situ on Prognosis of T1a,bN0 Breast Cancer. J Cancer. 2017;8(12):2328-2335. Available from: https://link.springer.com/article/10.1245/s10434-015-4367-9
- Gómez HL, Castañeda CA, Vigil CE, et al. Prognostic effect of hormone receptor status in early HER2 positive breast cancer patients. Hematol Oncol Stem Cell Ther. 2010;3(3):109-15. Available from: https://www.sciencedirect.com/science/article/pii/S1658387610500207
- Wuerstlein R, Harbeck N. Neoadjuvant Therapy for HER2-positive Breast Cancer. Rev Recent Clin Trials. 2017;12(2):81-92. Available from: https://www.ingentaconnect.com/content/ben/rrct/2017/00000012/00000002/art00006
- Hendrick RE, Helvie MA, Monticciolo DL. Breast Cancer Mortality Rates Have Stopped Declining in U.S. Women Younger than 40 Years. Radiology. 2021 Apr;299(1):143-149. Available from: https://pubs.rsna.org/doi/abs/10.1148/radiol.2021203476
- Johnson RH, Chien FL, Bleyer A. Incidence of Breast Cancer With Distant Involvement Among Women in the United States, 1976 to 2009. JAMA.2013;309(8):800–805. Available from: https://jamanetwork.com/journals/jama/article-abstract/1656255
- Lian W, Fu F, Lin Y, et al. The impact of young age for prognosis by subtype in women with early breast cancer. Nature 2017; Published 14 September 2017 [online]; Available from: https://www.nature.com/articles/s41598-017-10414-x
- Monticciolo DL. Invited Commentary: The Challenges of Early-Onset Breast Cancer. Published 6 Jan 2022 [online]. Available from: https://pubs.rsna.org/doi/abs/full/10.1148/rg.210191

