Imaging Tumours of the Ovary – Part 1
Dr Pierre Vassallo
The ovary is the site of several different tumours because it contains many different cell types and due to its proximity to the fallopian tube.1 While most ovarian tumours are benign or borderline, ovarian cancer is the second most common gynaecologic malignancy and the fifth most common cause of death in women.2
The ovary is composed of a connective tissue stroma, which in premenopausal women, contains ovarian germ cells called oocytes. Oocytes vary in number based on the patient’s age. They are surrounded by a layer of granulosa cells and an outer layer of thecal cells. The surface of the ovary is lined by a special layer of epithelium known as ovarian surface epithelium; the proximal (fimbrial) end of the fallopian tube lies close to the ovarian surface epithelium.3
Despite having ligamentous attachments to the uterus, fallopian tube and pelvic wall, the ovary is mobile within the peritoneal cavity. These ligaments act as passageways for the spread of cancer.
Ovarian tumours can be broadly divided into four main groups: epithelial cell, germ cell, sex cord stromal and metastases.
Epithelial tumours are the most common group of ovarian tumours with benign serous cystadenoma being the most common tumour overall accounting for 30% of all ovarian neoplasms. On the other hand, high grade serous adenocarcinomas (HGSCs) are the most common subtype of malignant ovarian tumours, most commonly seen in women over 61 years of age and constituting the most common cause of death from ovarian cancer.
While a histologic diagnosis that is based on tissue biopsy is required for further management, diagnostic imaging provides important information on the most likely tumour type as well as tumour size and spread. This data is needed to guide pre-operative management including surgical planning and to monitor the impact of treatment.
Histologic Subtypes of Ovarian Tumours
Ovarian epithelial tumours do not contain cells that resemble any of those normally present in the ovary. Thus, ovarian epithelial tumours are not truly primary. There is considerable evidence that shows that serous epithelial cells slough off from the fimbriated end of the fallopian tube and enter the ovary through the site of rupture of its surface that occurs during ovulation; this collection of fimbrial epithelial cells inside the ovary is called an epithelial inclusion cyst.4 In keeping with the above, there is strong evidence that HGSCs arise from sloughing off of malignant cells from the fimbriated end of the fallopian tube.5
The origin of mucinous epithelial tumours is less well understood, but recent studies have suggested that they may originate from pluripotential cells present in ovarian teratomas or Brenner tumours.6
Endometroid and clear cell epithelial tumours appear to arise from retrograde menstruation within endometrial implants or endometriomas.
Germ cell and sex cord stromal tumours are true primary ovarian tumours that originate from primitive germ cells, sex cord and stromal cells of the ovary.
Metastases reach the ovary from other organs through haematogenous, lymphatic, or direct spread; they originate mostly from the gastrointestinal tract, breast, lungs, and contralateral ovaries.
Clinical Findings, Risk Factors and Treatment
Smaller benign ovarian tumours are often discovered incidentally during routine imaging studies. Larger malignant and mostly epithelial type tumours present with bloating, pelvic/abdominal pain, satiety and urinary urgency and frequency. Epithelial tumours are more common in older women, while germ cell tumours are more often seen in younger women.
Risk factors for epithelial tumours include older age, obesity, a history of colorectal cancers, and familial cancer syndromes (BRCA1, BRCA2, Lynch syndrome and Peutz-Jeghers syndrome) and fertility treatments. Smoking is a risk factor associated with mucinous tumours. A genetic predisposition is seen in up to 25% of ovarian cancers.
Since the risk for ovarian cancers is high in those with genetic predisposition, screening with transvaginal US and cancer antigen 125 (CA-125) as well as prophylactic salpingo-oophorectomy have been advocated.7
Treatment is based on tumour stage (International Federation of Gynaecology and Obstetrics – FIGO system). Large tumours are surgically debulked and then treated with adjuvant chemotherapy. Neo-adjuvant chemotherapy may be used with extensive disease to induce reduction in tumour size prior to surgery. Monitoring of CA-125 levels allows disease surveillance with most types of tumour.
Imaging Ovarian Tumours
US, MRI and CT are all useful and offer complementary information for detecting ovarian tumours and assessing their extent; it is normal to employ at least two of these modalities (usually US and MRI) in pre-operative management with CT being used for post-operative follow-up.
Transvaginal US is particularly useful as the first imaging modality and for assessment of smaller ovarian tumours, while MRI allows further tumour characterisation and visualisation of the extent of larger tumours. Transabdominal US allows preliminary assessment of the extent of large tumours, but MRI is the imaging modality of choice for large lesions.1 Colour and greyscale Doppler US is useful for assessing tumour vascularity and ovarian torsion that may occur secondarily with an ovarian mass.
Ovarian imaging should confirm the ovary as the organ of origin of the tumour and whether the tumour is most likely benign, borderline, or malignant.
Smaller tumours may show a rim or claw of normal ovarian tissue around them, which will confirm that they are of ovarian origin (Fig 1). With larger tumours, the ovary itself may not be visible; its ovarian origin can only be established through its proximity to the bladder, uterus and bowel (Fig 2). An ovarian vein seen entering the tumour on CT or MRI scans also helps to identify its ovarian origin.
MRI allows better soft tissue characterisation; it also helps confirm suspected ovarian torsion. CT is useful as a rapid and accurate test for detecting fat and calcification within the tumour and for monitoring the impact of therapy in reducing tumour size. CT is also useful for detecting complications such as bowel obstruction and rupture.1
US findings that point towards a malignant ovarian tumour include large tumour size, multilocular lesions, papillary projections, irregular inner wall or irregular septa, solid tumour components (Fig 3) and blood flow with the lesion (Fig 4). Patients with lesions that demonstrate these features should be referred for gynaecological consultation.
Indeterminate lesions, such as complex lesions or those whose origin cannot be identified with certainty, should be further assessed with MRI. The presence of fat, haemorrhage or fibrous components within an ovarian tumour can be detected with MRI.
For staging of ovarian cancer, CT is the examination of choice since bowel motion and respiratory artifacts tend to limit the usefulness of MRI particularly for detecting peritoneal metastases. Peritoneal metastases measuring less than 5mm in diameter are not visible on CT particularly when they lie on the bowel surface or on the mesentery. CT is particularly useful for pre-operative staging, for restaging after debulking surgery and to detect complications such as intestinal obstruction.8
Epithelial Tumours
Epithelial tumours are classified as benign, borderline, or malignant based on histologic features that include extent of epithelial cell proliferation, degree of nuclear atypia and the presence or otherwise of stromal invasion.
Benign epithelial tumours typically present as unilocular cysts lined by a single layer of epithelium (Fig 5). Borderline tumours do not show evidence of invasion but may exhibit peritoneal spread. Malignant epithelial tumours may be low or high grade; low grade tumours are thought to arise from borderline lesions, with low grade malignant tumours showing stroma invasion.
Serous epithelial tumours are the most common ovarian neoplasms followed by mucinous epithelial tumours. Benignand borderline serous epithelial tumours, and low grade serous adenocarinomas are considered different entities from high grade serous cystadenocarcinomas. While mucinous cystadenoma, borderline, and mucinous adenocarcinomas are considered to be a continuum of the same tumour class.
The distinguishing feature between epithelial inclusion cysts and unilocular serous cystadenomas is size, where the latter measures >1cm in diameter. In patients who are still ovulating, a unilocular cystic lesion measuring >1cm in diameter is indistinguishable from an ovulating follicle. In this situation, a repeat endovaginal pelvic US after 6 weeks should be performed. Persistence of unilocular cyst on follow-up imaging would distinguish a serous cystadenoma from a functional ovarian cyst (follicle).
Serous epithelial tumours are commonly bilateral, while mucinous tumours are mostly unilateral. Borderline and malignant serous tumours may show early peritoneal spread, while mucinous borderline and malignant tumours tend to remain confined to the ovary unless they rupture, which can lead to pseudomyxoma peritonei.
Imaging features of epithelial tumours that point towards a borderline or malignant tumour include mural nodules, papillary projections, enhancing non-fatty and non-fibrous solid components, septa that are 3mm or more in thickness and septal vascularity. Large lesions and the presence of peritoneal or nodal spread are also indicative of a borderline or malignant lesion. Distant organ metastases are more commonly seen with HGSCs (Fig 6).
Conclusion
The above article introduced important clinical and imaging facts relating to ovarian tumours and discussed mainly serous epithelial tumours, the most common histologic subtypes. Part 2 of this article will present mucinous epithelial, germ cell, and sex cord stromal tumours as well as metastases.
Figure Legends
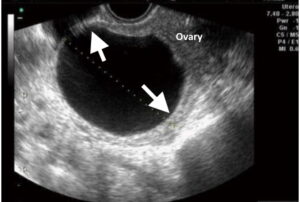
Figure 1. Transvaginal US image shows claws of normal ovarian tissue (arrows) surrounding a small ovarian tumour (serous cystadenoma).
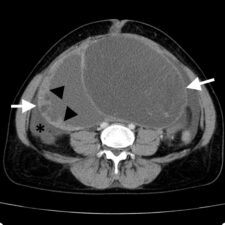
Figure 2. CT Scan showing a low grade serous carcinoma (LGSC) (white arrows) with thick cyst walls (arrowheads) and ascites (*).
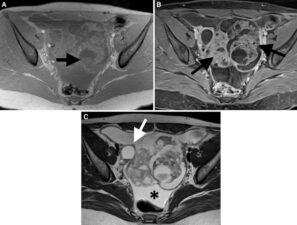
Figure 3. T1 weighted fat saturated pre- (A) and post-contrast (B) MRI images show a complex pelvic mass with solid components (arrows) that shows strong contrast enhancement (B). The T2 weighted image (C) depicts ascites (*) and peritoneal metastases (white arrow).
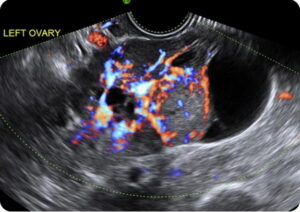
Figure 4. Transvaginal US of an LGSC appearing as complex ovarian tumour with abundant colour flow on Doppler imaging.
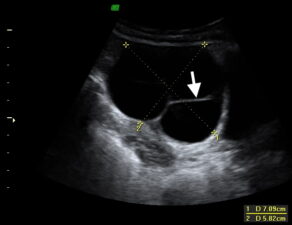
Figure 5. Transvaginal US showing a serous cystadenoma presenting as a unilocular cyst with thin walls; no mural nodules or papillary projections are evident. This tumour type may also present with multiple cysts; however these should exhibit thin walls (arrow) with no mural nodules, papillary projections, or septal vascularity.
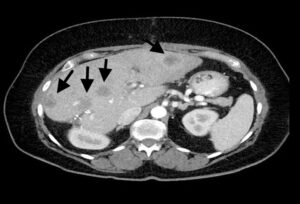
Figure 6: CT scan showing HGSC metastases in the liver (arrows).
References
- Taylor EC, Irshaid L, Mathur M. Multimodality Imaging Approach to Ovarian Neoplasms with Pathologic Correlation. Radiographics 2021;41(1):289-315.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7-30.
- Chhieng D, Hui P, eds. Cytology and Surgical Pathology of Gynecologic Neoplasms. New York: Spinger; 2011. .
- Klotz DM, Wimberger P. Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube? Arch Gynecol Obstet2017;296:1055–1062.
- Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 2017;8(1):1093.
- Ricci F, Affatato R, Carrassa L, et al. Recent Insights into Mucinous Ovarian Carcinoma. Int J Mol Sci2018;19: 1569.
- Rosenthal AN, Fraser L, Manchanda R, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol 2013;31(1):49-57.
- Jeong YY, Outwater EK, Kang HK. Imaging Evaluation of Ovarian Masses. Radiographics 2000; 20(5): 1445-1470

